Biologic therapy is a well-established strategy for managing moderate and severe psoriasis. Nevertheless, the high cost of such therapy, the relatively short span of clinical experience with biologics, and the abundance of literature now available on these agents have made evidence-based and consensus-based clinical guidelines necessary. The ideal goal of psoriasis treatment is to achieve complete or nearly complete clearing of lesions and to maintain it over time. Failing that ideal, the goal would be to reduce involvement to localized lesions that can be controlled with topical therapy. Although current evidence allows us to directly or indirectly compare the efficacy or risk of primary or secondary failure of available biologics based on objective outcomes, clinical trial findings cannot be directly translated to routine practice. As a result, the prescribing physician must tailor the treatment regimen to the individual patient. This update of the clinical practice guidelines issued by the Spanish Academy of Dermatology and Venereology (AEDV) on biologic therapy for psoriasis incorporates information from the most recent publications on this topic.
La terapia biológica representa una alternativa bien establecida en el manejo de la psoriasis moderada y grave. Sin embargo, su elevado coste, la experiencia relativamente limitada en su empleo clínico y la abundancia de publicaciones existentes hacen necesario el desarrollo de unas directrices basadas en la evidencia científica disponible y en el consenso de un grupo de expertos. El objetivo ideal del tratamiento de la psoriasis es conseguir y mantener a largo plazo un blanqueamiento completo o prácticamente completo o, en su defecto, una mínima afectación localizada y controlable con tratamientos tópicos. Aunque la evidencia disponible permite comparar de forma directa o indirecta la eficacia y las posibilidades de fracaso terapéutico primario o secundario de los diferentes fármacos según parámetros objetivos, las limitaciones en la extrapolación de los ensayos clínicos a la clínica diaria condicionan que la elección del fármaco y de la pauta de administración se realicen de forma individualizada en función de las características de cada paciente. La presente actualización de las directrices para el tratamiento de la psoriasis con agentes biológicos de la Academia Española de Dermatología y Venereología (AEDV) incorpora la información más reciente disponible a este respecto.
The Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology has launched a project to produce and update evidence-based guidelines for the systemic treatment of psoriasis with biologic agents. The purpose of these guidelines is to provide dermatologists with a treatment decision support tool based on the most recent scientific evidence that will help to ensure that patients with moderate to severe psoriasis receive the best possible treatment available. The guidelines will also serve as a useful reference for pharmacists, hospital managers, and the health authorities.
BackgroundPsoriasis is a chronic recurrent skin disease that affects 1.4% of the Spanish population, with geographical variations (1.2%-1.9%).1 In recent years, the association between psoriasis and a number of comorbidities has been recognized. As a result, psoriasis is now considered to be a systemic disease with predominantly cutaneous manifestations,2 which have a significantly negative impact on the patients’ quality of life3 and repercussions on their physical, emotional, sexual, and financial wellbeing as well as on their working life.
A pathogenetically related comorbidity that deserves special attention is psoriatic arthritis, a frequently disabling inflammatory arthropathy that appears on average 10 years after the diagnosis of psoriasis. Psoriatic joint disease develops in between 6% and 26% of patients with psoriasis, depending on the population studied.4 In the first epidemiological study carried out in Spain, 13% of a population of 3320 patients with moderate to severe psoriasis were found to have a confirmed diagnosis of psoriatic arthritis.5
To define the severity of psoriasis in clinical practice dermatologists generally use either the percentage of affected body surface area (BSA), a method that uses the palm of the hand as a measure of 1% of total BSA, or the Psoriasis Area and Severity Index (PASI).6,7 Other instruments that have been used to assess the severity of psoriasis and the efficacy of therapeutic interventions are the Physician's Global Assessment (PGA) and quality-of-life instruments, such as the Dermatology Life Quality Index (DLQI). The PGA correlates closely with the PASI, but the PASI is a better validated score and is the instrument of choice for measuring response in clinical trials.8 Worldwide, the DLQI is the most frequently used index for assessing health-related quality of life in dermatology. While easy to use and sensitive to change, this index is limited by its one-dimensional structure and variable cross-cultural equivalence.9
Measurement of visible skin involvement is essential for the accurate assessment of response in clinical trials, but in many cases such a measurement is not an appropriate criterion for severity because it does not take the patient's needs into account. Consensus has been reached in Spain on an operative definition of moderate to severe psoriasis as that presented by patients who are candidates for phototherapy or systemic therapy.10
A number of systemic therapies have been approved for use in the treatment of moderate to severe psoriasis: phototherapy (UV-A, UV-B, and narrowband UV-B radiation), photochemotherapy (psoralen plus UV-A radiation), traditional systemic agents (ciclosporin, methotrexate, and acitretin), and biologic agents (adalimumab, etanercept, infliximab, and ustekinumab). These treatments can be used in monotherapy or combined with topical treatments or each other, although some combinations may not be suitable because they could increase the risk of renal, hepatic, or metabolic toxicity and immunosuppression. The choice of therapy must be based on extensive clinical experience on the part of the prescribing dermatologist, and should always take into account the individual characteristics of the patient and the course and stage of disease.
Biologic treatments are designed to block specific molecular targets that intervene in the pathogenesis of psoriasis. Their favorable efficacy-to-risk ratio has been demonstrated in numerous clinical trials and postmarketing studies in patients with psoriasis and in other indications.
JustificationThe high cost of biologic drugs, the relatively limited experience with these agents in clinical use, and the extensive literature on this topic have generated a need for evidence-based guidelines developed through the consensus of an expert group. The ultimate aim should be to ensure that patients obtain the maximum therapeutic benefit from these treatments and to provide guidelines for safe and effective prescribing by the dermatologist. Several Spanish and European guidelines have already been published on the management of psoriasis with biologic therapy.11–17 We believe that this update to the guidelines previously published in Spanish12 is justified by several developments—the withdrawal from the market of efalizumab, the introduction of ustekinumab, and the emergence of new scientific evidence—in addition to the criticism that has been directed at some of the existing guidelines.18
The present guidelines review current evidence on the efficacy and safety of adalimumab, etanercept, infliximab, and ustekinumab in the treatment of moderate to severe psoriasis, the criteria for selecting candidates for biologic therapy, and aspects relating to therapeutic management, such as start of treatment, clinical response, and treatment failure, as well as the maintenance, withdrawal, and resumption of treatment and the adjustments that may be required in each case.
MethodsThese guidelines were drawn up by dermatologists from the Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology (AEDV) who have particular expertise and experience in the treatment of moderate to severe psoriasis. The resulting document was reviewed before publication by all the members of the Spanish Psoriasis Group.
The authors consulted the latest editions of guidelines,11–17,19 systematic reviews,20–23 meta-analyses,24–31 and cost-effectiveness analyses32–36 on the subject of the treatment of psoriasis with biologic agents. They also reviewed the summary of product characteristics (SPC) for each of the biologic agents and searched the literature in the MEDLINE database and the Cochrane Library for clinical trials with adalimumab, etanercept, infliximab, and ustekinumab published before September 2012.37–40 The studies identified were evaluated according to previously established criteria17 to define the level of evidence and strength of recommendation in each case. Finally, a search of the literature was undertaken to complement the information obtained by other methods.
Updating and LimitationsThe present guidelines are based on the best information available at the time of writing, and this document will be updated regularly. The results of future studies may make it necessary to reconsider the conclusions and recommendations of these guidelines. The present document was drawn up to help dermatologists in the management of moderate to severe psoriasis with biologic drugs and is not intended to serve as a strict treatment guideline. Decisions concerning treatment must always be taken on a case-by-case basis with the sole aim of benefiting the patient; they should also be based on the SPC of each drug and take into account any pertinent pharmacoeconomic considerations.
Candidates for Treatment With Biologic AgentsBiologic therapy is generally indicated (according to the European Medicines Agency [EMA] SPCs) for the treatment of adult patients with moderate to severe plaque psoriasis who have failed to respond to other systemic therapies or who are intolerant to or have a contraindication to such therapy. In most of the clinical trials with biologic agents undertaken as part of the drug approval process and submitted to the pertinent regulatory bodies, the only inclusion criterion used was the presence of moderate to severe plaque psoriasis (PASI ≥ 10-12); the US Food and Drug Administration does not include the additional criteria specified by the EMA.
The AEDV consensus statement10 defines moderate to severe psoriasis as psoriasis that requires (or has previously required) systemic treatment, whether with conventional or biologic drugs, and/or phototherapy or photochemotherapy (Table 1).
Recommendations: Eligibility Criteria for Biologic Therapy.
| Systemic treatment (conventional or biologic) is indicated in patients with moderate to severe psoriasis, that is, psoriasis that meets any of the following conditions: a) not controlled with topical treatment; b) extensive involvement (BSA ≥ 5%-10%), PASI ≥ 10); c) rapid worsening; d) involvement of visible areas; d) functional impairment (palmoplantar, genital); e) subjective perception of severity (DLQI>10); f) extensive erythroderma or pustular psoriasis; or g) associated with psoriatic arthropathy10 (level of evidence 4, consensus). |
| According to the current summaries of product characteristics, biologic treatments are indicated for the treatment of moderate to severe plaque psoriasis in patients who have failed to respond to, or who have a contraindication to, or are intolerant (adverse effects or probable toxicity, whether acute or with a cumulative dose) to conventional systemic therapies, including acitretin, methotrexate, and ciclosporin, or to phototherapy, photochemotherapy, or other biologic agents10 (level of evidence 4, consensus). This definition includes the following groups: |
| 1. Patients in whom effective control is not achieved with the available systemic agents, in monotherapy or combination regimens |
| 2. Patients in whom relapses occur rapidly, that is, within less than 3 months after withdrawal of any type of treatment |
| 3. Patients who require high doses of conventional systemic therapies (with the corresponding risk of adverse effects due to acute or cumulative toxicity in a substantial percentage of patients) |
| 4. Patients who are intolerant to a systemic therapy (who experience toxicity or adverse reactions with effective doses) or who have a high risk of cumulative toxicity with methotrexate, ciclosporin, acitretin, phototherapy, or photochemotherapy even when laboratory test results are normal. The risk of toxicity may be due to the dose required, the duration of treatment, individual susceptibility, or to individual risk factors, such as age, sex, comorbidity, or potential drug interactions |
| 5. Patients who are not good candidates for treatment with phototherapy or photochemotherapy because of their work, daily schedule, travel requirements, or availability |
Abbreviations: BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index.
The choice of biologic therapy should be guided by the criteria established in the SPC for each drug and the relevant treatment guidelines written by groups of experts. Treatment should always be individualized taking into account factors such as the presence of concomitant diseases, joint involvement, the patient's age, weight, life expectancy, and lifestyle as well as the risk of adverse effects. The prescribing physician should also evaluate the history of the patient's disease (including previous therapy) and the severity of psoriasis at the time of prescription.
All the biologic agents approved for the treatment of psoriasis should be made available to all patients who are candidates for such treatment, without unnecessary delay or any type of limitation that might represent unequal treatment of the patient.
Prescribers of Biologic Therapy to Patients With PsoriasisBiologic agents should be prescribed by dermatologists with experience in the treatment of psoriasis with systemic agents. Special emphasis must be given to the desirability of assessing and documenting disease severity (PASI, BSA, PGA, DLQI, etc.) so that clinical response can be assessed in each patient and the choice of treatment justified in the case of a prescription audit.
Treatment PhasesThe systemic treatment of psoriasis with biologics is divided into 2 phases: induction and maintenance. Typically, the induction phase corresponds to the period of treatment up to week 16, although—depending on the drug and dosage—it can be prolonged to up to 24 weeks, the point at which the efficacy of all biologics tends to plateau.41,42 Maintenance therapy is started once induction is complete, and the regimen prescribed must take into account the particular characteristics of long-term biologic treatment.
Definition of Treatment FailureTreatment failure is a consideration of great importance in biologic therapy because it generally requires a change of biologic agent and increases the cost of treatment (the cost of acquiring the initial agent plus the difference between the cost of acquiring the induction-phase doses of the replacement and the maintenance regimen of the initial treatment).42
Primary treatment failure is defined as the failure during the induction phase to obtain a 50% improvement in PASI from baseline (PASI 50)—known as the efficacy threshold.14,43 Although the lack of a PASI 50 response is the key criterion for treatment failure, this decision must be confirmed on a case-by-case basis jointly by the physician and the patient, taking into account the subjective impact on the patient of the benefit achieved, prior treatment history, and the treatment alternatives available.
Secondary treatment failure is defined as loss of the PASI 50 response during the maintenance phase, although other definitions can be established based on an absolute PASI score (5), the PGA, or a combination of PASI and a quality-of-life score.10 In the opinion of the members of the Spanish Psoriasis Group, an absolute PASI score above 5 or a PGA score above 2 would be considered criteria for treatment failure in most patients, but the patient's opinion must also be taken into account.
The EMA SPCs37–40 for each biologic agent specify different time points at which a decision must be taken on whether to discontinue treatment because of treatment failure: 12 weeks after start of treatment for etanercept; 14 or 22 weeks for infliximab42; 16 weeks for adalimumab; and 28 weeks (before the fourth injection) for ustekinumab. In clinical practice, these differences are rarely taken into account, and the decision regarding the success or failure of treatment is taken for all 4 drugs between weeks 16 and 24 of the induction phase41 and usually before prescribing the next injection or box of pills.
Action in the Case of Primary or Secondary Treatment FailureWhen the outcome of treatment with the chosen biologic agent is unsatisfactory (primary or secondary treatment failure), the possible courses of action that may be followed to improve the results are a) to intensify treatment, b) to switch to another biologic drug, or c) to start a combination regimen.
Although not explicitly approved in the SPC, treatment intensification (increasing the dose or shortening the interval between doses) is a strategy that has been used during both clinical trials and open-label extension trials for all the currently available biologics.44,45 Temporary dose escalation has been proposed as a strategy in patients with primary or secondary treatment failure. Leonardi et al.46 temporarily increased the dosage of adalimumab in such patients to 40 mg/wk; at 12 weeks and 24 weeks after dosage escalation, 27% and 38%, respectively, of the patients had achieved PASI 75 or returned to the original dosage regimen of 40mg every 2 weeks. The patients most likely to benefit from dosage escalation (48%) were those with secondary treatment failure who had relatively low weight and a short disease duration. In a prospective observational cohort of patients whose response to treatment with adalimumab was unsatisfactory, a PASI 50 response was achieved by 25% of patients at week 12 and 35% at week 24 following intensification of the adalimumab treatment regimen. In the group in which methotrexate was added to the original adalimumab regimen and the dose of the biologic was not increased, the corresponding percentages were 9% and 18%, respectively.47
In the PHOENIX 2 open-label extension study of ustekinumab, patients were offered the choice, with the agreement of their physician, of changing their therapeutic regimen by reducing the interval between doses from 12 to 8 weeks and/or by switching from 45 to 90 mg per dose. In total, 28% of the patients followed the more intense regimen between weeks 52 and 244.48 Of the 454 patients who opted to change their regimen, 51% (230) had already achieved a PASI 75 and 12% (55) a PASI 90 response when the adjustment was made. Most (70%-80%) of the patients who had a PASI response of less than 75% when the regimen was changed subsequently achieved and maintained a PASI 75 response for up to 3 years.
Although intensification of treatment has not been studied specifically with infliximab in psoriasis patients, the strategy is often used in clinical practice by reducing the interval between infusions from 8 weeks to 6 or even 4 weeks.49
The SPC for etanercept recommends treatment with a dosage of 50 mg/wk from 12 weeks onwards; however, the findings of the open-label extension study indicate that some patients who lose response after the induction phase can regain the response by continuing with a dosage of 100 mg/wk.50
The alternative strategy (switching to another biologic agent) is preferable from the pharmacoeconomic standpoint.51
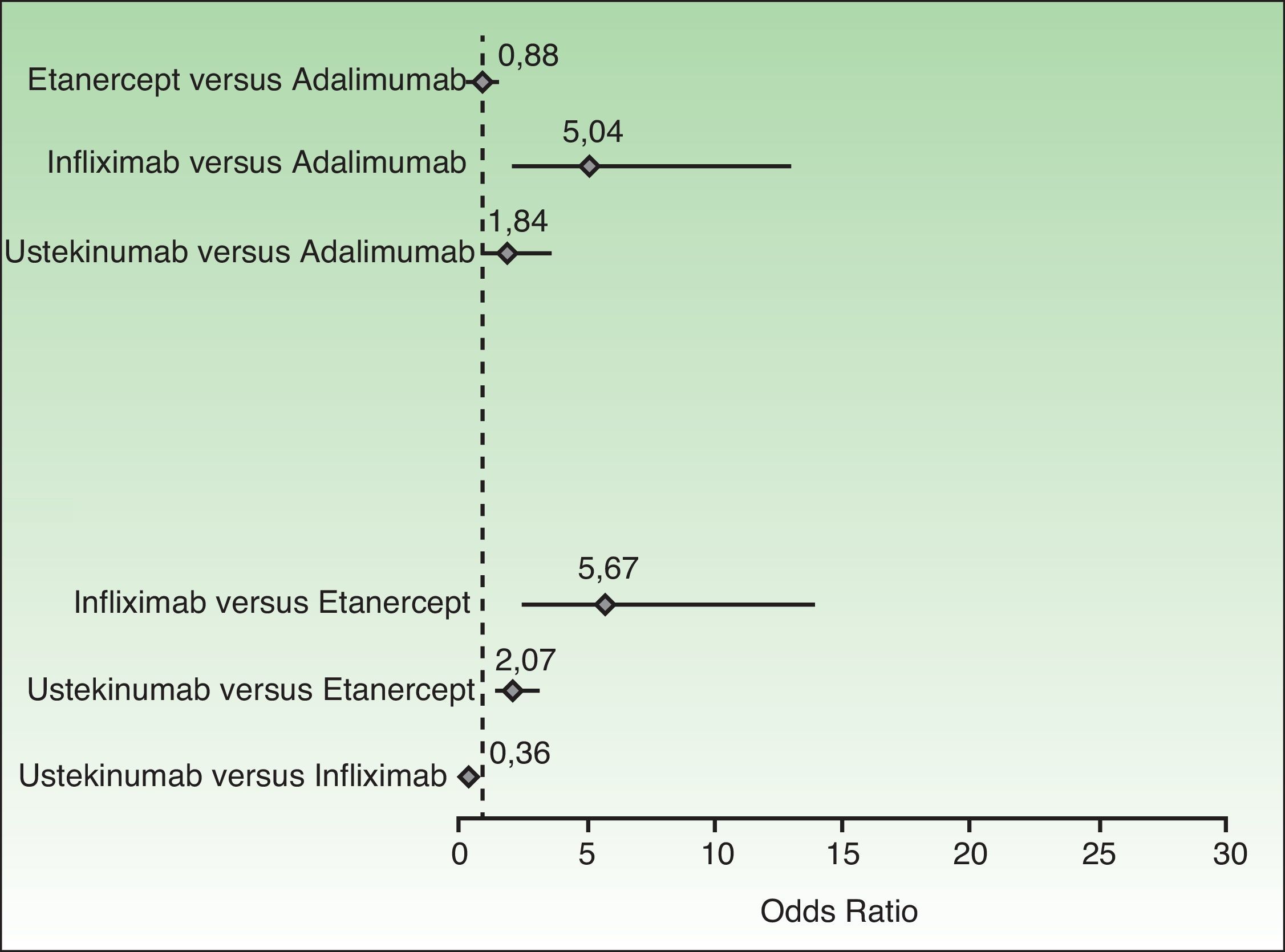
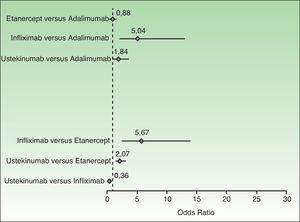
When considering a switch to a different biologic agent because of primary or secondary treatment failure, the prescriber must bear in mind numerous considerations, including the results of pairwise comparisons of PASI 75 response rates (Fig. 1), the presence or absence of active joint disease, the clinical characteristics of the patient (weight, risk of infection, comorbidities, and contraindications related to specific biologic agents), the mechanism of action, the appropriateness of the dosage and route of administration, the additional cost of induction therapy, and other pharmacoeconomic considerations.
Random-effects model showing pairwise comparisons of PASI 75 response rates to biologic agents. The diamonds represent the odds ratios and the horizontal lines the 95% CIs. PASI 75 indicates a 75% reduction in Psoriasis Area and Severity Index score over baseline.
Adapted from Lin et al.30
The same considerations should be taken into account when considering a change because of an adverse event, although in such cases particular importance should be given to the relative risk of infections and immunogenicity, and other aspects, such as the possible association between a specific drug or class of drugs and paradoxical reactions or autoimmune responses.
Current evidence is insufficient to support definitive recommendations about which biologic is preferable in situations of primary or secondary failure of biologic therapy. In cases of primary or secondary failure after 3 to 6 months of treatment with etanercept, a PGA score of less than 2 was reported in 49% of patients who switched to adalimumab for 16 weeks52 and in 65% of those treated with infliximab for 10 weeks53; in the case of patients treated with ustekinumab 90 mg, 49% achieved a PASI 75 response 12 weeks after the switch.54 There is also evidence indicating a good response after a switch from infliximab to etanercept55 or from a tumor necrosis factor (TNF) inhibitor to ustekinumab.56
The available evidence would suggest that the response rate for etanercept, ustekinumab, and adalimumab is somewhat lower in patients previously treated with another biologic agent while response to infliximab is not influenced by prior biologic therapy.19 However, the evidence is insufficient to establish the ideal order of preference when a biologic drug is being considered as second-line therapy in cases of primary or secondary failure following treatment with another biologic agent.
Combination therapy is often used to improve the efficacy of a biologic regimen, even in the absence of treatment failure. This strategy is discussed below in a separate section.
Therapeutic EfficacyThe ideal outcome for psoriasis treatment is to achieve and maintain in the long term complete or almost complete clearing (PGA≤1, with minimum BSA involvement) or, failing that, a minimal and localized affected area that can be controlled with topical therapy (PGA≤2, PASI<5).10
The therapeutic goal during the induction phase is to achieve at least a PASI 75 response and an optimal response is PASI 90 or better (absence of clinical signs, clearing, or only minimal signs of disease) and an absolute PASI of less than 5 or even less than 310; absolute PASI score is already being used as a criterion in clinical practice to assess the long-term efficacy of biologic therapy.57 In patients with a baseline PASI of 20 or less, a PASI 75 response may not be an adequate treatment goal in the long term (beyond 6 months) because the absolute PASI score obtained would be above 5.
When the response is less than PASI 75, treatment should be modified if the patient is not satisfied or if the disease is having a significant impact on the patient's quality of life (DLQI > 5).41 A response of less than PASI 50 is considered to be a treatment failure (primary during the induction phase or secondary if a satisfactory response had already been achieved) or relapse (if treatment has been discontinued). In the case of primary or secondary failure, patients should generally be switched to an alternative treatment. However, achieving a 50% improvement in PASI can be regarded as a satisfactory response in selected patients for whom other alternatives do not exist and other treatments are not effective or are contraindicated.
Meta-analyses of efficacy parameters based on patient evaluation (patient-reported outcomes) and non-PASI outcomes do not adequately differentiate between the biologic agents currently available for the treatment of psoriasis.58 If the achievement of a DLQI score of 0 or 1 is used as a therapeutic goal, a PASI 90 response or better correlates much more closely with this objective than a PASI 75 response,59 and the improvement in DLQI compared to baseline is also higher in patients with an optimal response.60
In most patients and for all of the biologic agents used to treat psoriasis, maximal therapeutic efficacy is achieved within 6 months. The therapeutic goal of maintenance therapy should be to maintain effectiveness in the long term. To do this, the clinician must consider possible adjustments of the dose or treatment intervals whenever necessary, as well as combinations with other therapies (topical treatments, phototherapy, or systemic drugs) and, if appropriate, take steps to modify factors such as obesity or a sedentary lifestyle. The outcome of any therapeutic intervention must be assessed within a maximum of 3 months.
Choosing a Biologic AgentThe most important factor in the choice of a biologic agent or the calculation of its cost/benefit ratio is the efficacy of the drug relative to baseline, assessed on the basis of scientific evidence from clinical trials. The criteria most often used for comparison are the percentages of patients achieving PASI 75, PASI 90, and PASI 50 for each of the available drugs. However, it is important to bear in mind that patient populations in clinical practice are very different from those of clinical trials61 and that while response rates may allow us to infer the response of populations, they cannot predict individual patient response.
When assessing clinical trials of biologic agents during the drug approval process, the health authorities have generally considered the percentage of patients achieving a PASI 75 response to be the most relevant parameter for judging efficacy.54,62–81 It is also the primary outcome used in most meta-analyses.25–30,36 However, the point at which this response (the primary outcome) is assessed is different for each biologic agent and depends on the trial design. Meta-analysis of the effect at 24 weeks has been proposed as a method for achieving a head-to-head comparison of all the drugs available31 because 24 weeks is usually the end of the therapeutic response-induction phase. For all the biologics used in psoriasis, PASI 75 response rates reach their peak at 24 weeks and after this point the percentage of responders stops rising.42 When the duration of placebo treatment is under 24 weeks, the last observation can be carried forward (LOCF).
Some studies directly comparing biologic agents have been published.54,68,69 Of these, the most important is the ACCEPT study because it compares 2 biologic agents currently available on the market.54 In that study, a PASI 75 response was observed at week 12 in 72% of the patients treated with ustekinumab 45 mg (patients weighing 100 kg or less) and in 65% of patients treated with ustekinumab 90 mg (patients weighing over 100 kg), compared to 57% in the group receiving etanercept 50 mg twice a week.40 The decision to use 12 weeks as the time point for comparison of effect in that study has been criticized because the timing does not permit evaluation of the maximum response for either etanercept18,82 or ustekinumab.76,77 Moreover, the absence of a placebo control group represents an additional methodological problem for the purposes of meta-analysis.
For the reasons mentioned above, PASI 90 response rates have particular clinical relevance as therapeutic targets, especially when this response is achieved after the end of the induction phase (16-24 weeks) of therapy. A PASI 90 response is especially important from the clinical standpoint since it usually implies an absolute PASI score of less than 3, equivalent to complete or almost complete clearance, and this result is usually considered satisfactory by both the patient and the dermatologist.
The PASI 50 response rate is also important, since not achieving or not maintaining such a response represents treatment failure (primary and secondary, respectively), making it necessary to switch to a new biologic, initiate a combination regimen, or intensify treatment.
When the PASI 75 response of different biologics is the same, the drug offering the best PASI 50 response rate should be preferred; in other words, the best choice is the option with the lowest rate of primary treatment failure. The lower the primary failure rate, the lower the likelihood of having to switch to another treatment during the induction phase. Switching has a counterproductive effect psychologically and involves lost time as well as higher costs because the pharmacokinetic loading dose during the induction phase of treatment is more expensive than the maintenance regimen. Adalimumab is the biologic with the lowest additional cost during induction therapy with respect to the maintenance phase (18% at 16 weeks).51
The prescribing physician must take all these considerations into account. Tables 2–5 show comparisons based on recent meta-analyses of the PASI 50, PASI 75, and PASI 90 response rates measured at the time of assessment of the primary endpoint. Table 6 shows the results of the meta-analysis of efficacy at 24 weeks (using LOCF where necessary).31 A limitation of the published meta-analyses is that the results for ustekinumab have been calculated on the basis of data corresponding to all the doses used in clinical trials rather than only the data corresponding to the patients treated with the doses specified in the SPC, namely 45 mg for those weighing 100 kg or less and 90 mg for those weighing more than 100 kg.
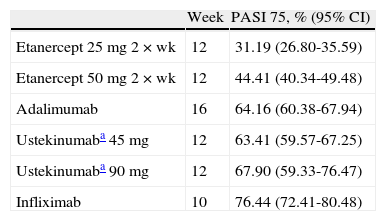
Incremental Efficacy (%) Measured at the Time of Assessment of the Primary Response Variable in Each Trial According to the Meta-Analysis by Ferrándiz et al36
| Week | PASI 75, % (95% CI) | |
| Etanercept 25 mg 2×wk | 12 | 31.19 (26.80-35.59) |
| Etanercept 50 mg 2×wk | 12 | 44.41 (40.34-49.48) |
| Adalimumab | 16 | 64.16 (60.38-67.94) |
| Ustekinumaba 45 mg | 12 | 63.41 (59.57-67.25) |
| Ustekinumaba 90 mg | 12 | 67.90 (59.33-76.47) |
| Infliximab | 10 | 76.44 (72.41-80.48) |
Abbreviations: PASI, Psoriasis Area and Severity Index.
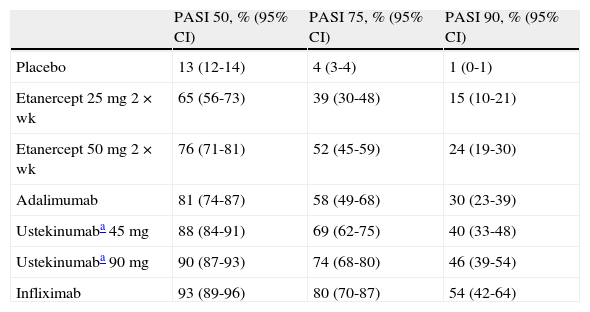
Efficacy of Biologics (Probability of Response Expressed as a Percentage) Measured at the Time of Assessment of the Primary Response Variable According to the Network Meta-Analysis by Reich et al29
| PASI 50, % (95% CI) | PASI 75, % (95% CI) | PASI 90, % (95% CI) | |
| Placebo | 13 (12-14) | 4 (3-4) | 1 (0-1) |
| Etanercept 25 mg 2×wk | 65 (56-73) | 39 (30-48) | 15 (10-21) |
| Etanercept 50 mg 2×wk | 76 (71-81) | 52 (45-59) | 24 (19-30) |
| Adalimumab | 81 (74-87) | 58 (49-68) | 30 (23-39) |
| Ustekinumaba 45 mg | 88 (84-91) | 69 (62-75) | 40 (33-48) |
| Ustekinumaba 90 mg | 90 (87-93) | 74 (68-80) | 46 (39-54) |
| Infliximab | 93 (89-96) | 80 (70-87) | 54 (42-64) |
Abbreviations: PASI, Psoriasis Area and Severity Index.
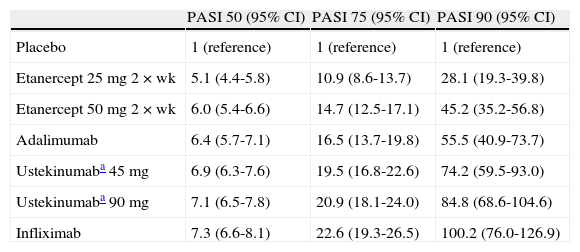
Efficacy (Relative Risk vs Placebo) Measured at the Time of Assessment of the Primary Response Variable in Each Trial According to the Network Meta-Analysis by Reich et al29
| PASI 50 (95% CI) | PASI 75 (95% CI) | PASI 90 (95% CI) | |
| Placebo | 1 (reference) | 1 (reference) | 1 (reference) |
| Etanercept 25 mg 2×wk | 5.1 (4.4-5.8) | 10.9 (8.6-13.7) | 28.1 (19.3-39.8) |
| Etanercept 50 mg 2×wk | 6.0 (5.4-6.6) | 14.7 (12.5-17.1) | 45.2 (35.2-56.8) |
| Adalimumab | 6.4 (5.7-7.1) | 16.5 (13.7-19.8) | 55.5 (40.9-73.7) |
| Ustekinumaba 45 mg | 6.9 (6.3-7.6) | 19.5 (16.8-22.6) | 74.2 (59.5-93.0) |
| Ustekinumaba 90 mg | 7.1 (6.5-7.8) | 20.9 (18.1-24.0) | 84.8 (68.6-104.6) |
| Infliximab | 7.3 (6.6-8.1) | 22.6 (19.3-26.5) | 100.2 (76.0-126.9) |
Abbreviations: PASI, Psoriasis Area and Severity Index.
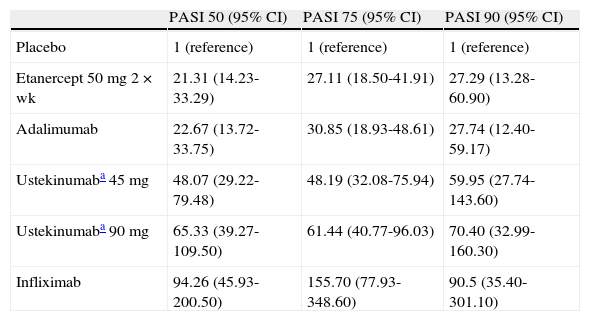
Efficacy (Mean Odds Ratio and 95% CI vs Placebo) Measured at the Time of Assessment of the Primary Response Variable in Each Trial According to the Bayesian Network Meta-Analysis by Lin et al30
| PASI 50 (95% CI) | PASI 75 (95% CI) | PASI 90 (95% CI) | |
| Placebo | 1 (reference) | 1 (reference) | 1 (reference) |
| Etanercept 50 mg 2×wk | 21.31 (14.23-33.29) | 27.11 (18.50-41.91) | 27.29 (13.28-60.90) |
| Adalimumab | 22.67 (13.72-33.75) | 30.85 (18.93-48.61) | 27.74 (12.40-59.17) |
| Ustekinumaba 45 mg | 48.07 (29.22-79.48) | 48.19 (32.08-75.94) | 59.95 (27.74-143.60) |
| Ustekinumaba 90 mg | 65.33 (39.27-109.50) | 61.44 (40.77-96.03) | 70.40 (32.99-160.30) |
| Infliximab | 94.26 (45.93-200.50) | 155.70 (77.93-348.60) | 90.5 (35.40-301.10) |
Abbreviations: PASI, Psoriasis Area and Severity Index.
Results of Incremental Efficacy (Risk Difference Expressed as a Percentage) at 24 Weeks in the Meta-Analysis by Lucka et al31
| PASI 75, % (95% CI) | |
| Etanercept 25 mg 2×wk | 45 (34-56) |
| Etanercept 50 mg 2×wk for 12 wk and 25 mg 2×wk thereafter | 50 (42-57) |
| Etanercept 50 mg 2×wk | 62 (52-72) |
| Adalimumab | 60 (45-74) |
| Ustekinumaba 45 mg | 70 (64-77) |
| Ustekinumaba 90 mg | 77 (71-83) |
| Infliximab | 78 (72-83) |
Abbreviations: PASI, Psoriasis Area and Severity Index.
The results are expressed as probability of response, risk difference or incremental efficacy (the difference vs placebo in response rates), relative risk, or odds ratio. It is important to understand the difference between relative risk and odds ratio. If the PASI 75 response rate for a given biologic is 75%, a patient is 3 times (75/25) more likely to achieve this rate than not, so the odds are 3 to 1. If the PASI 75 for placebo is 5%, the likelihood (odds) of a patient achieving the target response are 5/95, or 0.0526. The relative risk (drug vs placebo) of a patient achieving a PASI 75 response is 15 (75/5) and the odds ratio is 57 (75×95/25×5).
When interpreting these tables, note that if the 95% CI intervals of 2 biologic agents overlap the difference in efficacy between them is not statistically significant.
Finally, Fig. 1 shows the results of a bilateral comparison based on a Bayesian network meta-analysis30; when the 95% CI, which in this type of meta-analysis is referred to as the credible interval (95% CrI), does not touch or cross the 0 value of the odds ratio on the x axis, the first member of the pair can be said to be superior (if the 95% CrI is to the right of the 0 value) or inferior (if the 95% CrI is to the left).
Other Selection CriteriaWhen choosing between biologic agents, the clinician must take into account many factors relating to the characteristics of the patient, the disease, and the therapies under consideration. An important aspect relating to the patient are the comorbidities that may represent a relative contraindication in the case of anti-TNF agents, such as advanced congestive heart failure, lupus erythematosus and other autoimmune diseases, as well as the presence of demyelinating disease.83 In such cases, ustekinumab would be the first-line choice among biologic agents.
Some of the evidence suggests that the risk of certain infections is somewhat lower with etanercept than with other anti-TNF agents,84,85 but the evidence is insufficient to allow us extrapolate possible conclusions (or recommendations) for patients with psoriasis. Although the evidence is limited, there is more published experience with etanercept in the treatment of patients who have chronic infection with hepatitis C virus or human immunodeficiency virus (HIV); the choice of a biologic agent in such patients should be decided on a case-by-case basis and biologic therapy must be closely monitored by both the dermatologist and the physician in charge of managing the chronic infection.86,87
In the case of pediatric patients, etanercept is the only biologic approved for the treatment of psoriasis in children over 6 years of age.
In women of childbearing potential, each case should be considered individually, taking into account the severity of psoriasis and the patient's opinion. In general, exposure of a fetus from the second trimester of pregnancy onwards (when antibodies can cross the placenta) should be avoided. The elimination half-life of each biologic agent must be taken into account in such cases. If treatment is discontinued when the patient detects her pregnancy, it is highly unlikely that any fetal exposure will occur.88
In obese patients weighing more than 90 or 100 kg somewhat lower response rates should be expected (in particular in terms of the optimal response). In this subpopopulation, primary failure rates are higher when a fixed dose of the biologic agent is prescribed88 and the higher cost associated with weight-dependent treatment in the case of infliximab or ustekinumab should be borne in mind.
With respect to disease-related factors, the clinician must take into account the presence or absence of psoriatic arthritis the baseline severity of disease (PASI score), and the need for a more or less rapid response to treatment. In principle, although the effect of ustekinumab on psoriatic arthritis has been demonstrated,89 it should be noted that, at present, only anti-TNF agents have been approved for this indication. In the absence of direct comparison studies, meta-analysis has not shown any significant differences between these agents with respect to their effectiveness as measured using parameters such as the American College of Rheumatology 20% improvement criteria (ACR20), the ACR50, and the ACR70.90 Most studies have been conducted in patients with the polyarticular form of psoriatic arthritis, and fewer data are available on efficacy with respect to the axial component in this subgroup. There is, however, evidence supporting the efficacy of anti-TNF agents and ustekinumab on enthesitis and dactylitis.90
Baseline severity (PASI score) does not a priori determine the choice of biologic agent, and there is no scientific evidence supporting the restriction of a particular biologic to patients with more severe psoriasis or as a second-line biologic treatment.28 The following specific profile has been proposed for the patient most suited to treatment with infliximab91: severe psoriasis, especially when associated with onychopathy or psoriatic arthritis, when clearance is urgent, poor patient compliance with self-medication is expected, and long-term continuous treatment is likely to be necessary. There is, however, no reason to restrict the use of infliximab to patients with PASI scores above 20: no significant differences in the PASI 75 response rate have been demonstrated in association with baseline PASI scores above and below 20.72 Neither does the mechanism of action of ustekinumab, which is not a TNF inhibitor, justify its relegation to being a second-line biologic.
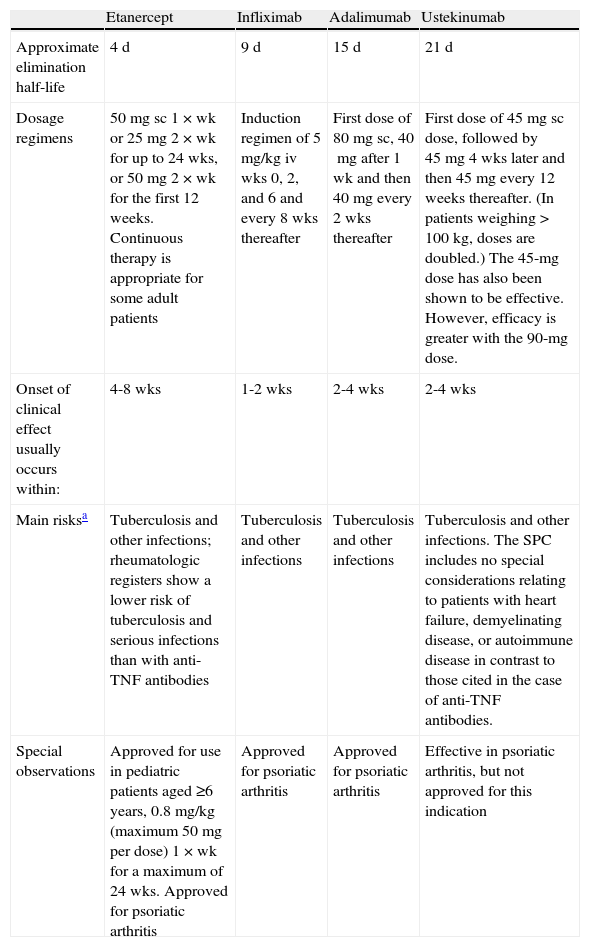
Differences in pharmacokinetics, mechanism of action, and safety profiles between the available biologic agents must all be taken into account (Table 7). All of these factors make it difficult to categorize biologics into therapeutic classes grouping interchangeable treatments.92 Consideration should also be given to the route and convenience of administration and relative differences in the cost of induction therapy.51
Main Pharmacokinetic Dosage and Safety Parameters of Biologics in the Treatment of Moderate to Severe Psoriasis.
| Etanercept | Infliximab | Adalimumab | Ustekinumab | |
| Approximate elimination half-life | 4 d | 9 d | 15 d | 21 d |
| Dosage regimens | 50 mg sc 1×wk or 25 mg 2×wk for up to 24 wks, or 50 mg 2×wk for the first 12 weeks. Continuous therapy is appropriate for some adult patients | Induction regimen of 5 mg/kg iv wks 0, 2, and 6 and every 8 wks thereafter | First dose of 80mg sc, 40mg after 1 wk and then 40mg every 2 wks thereafter | First dose of 45 mg sc dose, followed by 45 mg 4 wks later and then 45 mg every 12 weeks thereafter. (In patients weighing >100 kg, doses are doubled.) The 45-mg dose has also been shown to be effective. However, efficacy is greater with the 90-mg dose. |
| Onset of clinical effect usually occurs within: | 4-8 wks | 1-2 wks | 2-4 wks | 2-4 wks |
| Main risksa | Tuberculosis and other infections; rheumatologic registers show a lower risk of tuberculosis and serious infections than with anti-TNF antibodies | Tuberculosis and other infections | Tuberculosis and other infections | Tuberculosis and other infections. The SPC includes no special considerations relating to patients with heart failure, demyelinating disease, or autoimmune disease in contrast to those cited in the case of anti-TNF antibodies. |
| Special observations | Approved for use in pediatric patients aged ≥6 years, 0.8 mg/kg (maximum 50 mg per dose) 1×wk for a maximum of 24 wks. Approved for psoriatic arthritis | Approved for psoriatic arthritis | Approved for psoriatic arthritis | Effective in psoriatic arthritis, but not approved for this indication |
Abbreviations: iv, intravenous; sc, subcutaneous; SPC, summary of product characteristics.
In principle, biologic therapy in patients with psoriasis is continuous, making it particularly suitable for patients who experience rapid relapse on withdrawal of any type of treatment. In some situations, withdrawal of treatment may be considered advisable for various reasons, such as surgery, pregnancy, travel, or the patient's own decision. Intermittent therapy with biologic agents may be considered in selected patients who experience periodic flares of psoriasis that do not respond to conventional systemic treatments or in whom such treatments are contraindicated. This is discussed for each biologic in the corresponding section.
In patients who maintain an optimal response in the long term (> 1 year), reduction of the dose or frequency of administration can be considered.44 Cessation of treatment may be discussed with the patient depending on the prescribing physician's assessment of the case.
Long-term Treatment: Continuous, Intermittent, or As NeededThe open-label extensions of some clinical trials have provided evidence of long-term efficacy.
In the REVEAL study (adalimumab), patients who had a PASI 75 response at weeks 16 and 33 maintained that response for 100 weeks in 83% of cases and for 160 weeks in 76% (LOCF); the corresponding percentages for a PASI 90 response were 59% and 50%, respectively.93 While many of the patients who achieve only the minimum response required to be categorized as responders during induction therapy maintain this response with sustained continuous treatment, patients who achieve the highest responses (PASI 90 or PASI 100) during the induction phase are more likely to maintain the response thereafter.
In the PHOENIX 1 study (ustekinumab), initial PASI 75 responders were re-randomized at week 40 to continue on a regimen of treatment every 12 weeks; the patients in that group generally maintained the initial response rate through week 244 (5 years). At week 244, the PASI 75 response rate in this group of patients for doses of 45 mg and 90mg was 79% and 81%, respectively; the corresponding rates for PASI 100 were 31% and 38%.94
In a post hoc analysis that included patients treated with etanercept at different doses over a period of up to 4 years, 67% of those who responded satisfactorily during the induction period (PGA 0/1) maintained the response at 24 weeks, and with only slight variation through week 48.95
Data on long-term outcomes with infliximab are scarce and determined by the duration of clinical trials (< 1 year) because no data from open-label extension trials is available. In the EXPRESS II study, 74% of the patients who achieved a PASI 75 response during the first few weeks maintained this response until week 50 with continued treatment.72
Data from the records of patients with psoriasis indicate that the overall efficacy of anti-TNF agents decreases over time since the number of those who adhere to the treatment declines because of loss of efficacy or adverse events; this is sometimes referred to as the retention rate or “drug survival”.45 In a study based on the Dermbio clinical database, the treatment retention rate at 4 years was 40% for adalimumab and etanercept and 70% for infliximab.45 However, this study did not take into account biases that were not evaluated, such as the criteria used by the dermatologists in the selection of the biologic agent and differences in the management of the drug regimens.
Very little data on adherence to ustekinumab treatment is available because the drug only recently came onto the market. In another study based on the Dermbio database, but limited to the first year of treatment (maximum follow-up of 321 days), adherence to ustekinumab was 95.5%, compared to 75% for the anti-TNF drugs as a group.96
Although biologic treatment is generally intended to be continuous and long-term, in selected cases intermittent or as needed treatment can be a valid option. In patients who show optimal response in the long term, withdrawal of treatment or a reduction in the dose and/or frequency of administration can be considered, although there is insufficient scientific evidence to define the ideal approach in each case.
In the case of adalimumab, data are available from the open-label extension phase of the REVEAL study. At week 33, PASI 75 responders at weeks 16 and 33 were re-randomized to 1 of 2 groups: in one group treatment was continued and in the other the biologic was discontinued and subsequently reintroduced if there was a loss of adequate response (defined as a <PASI 50 response relative to baseline or a 6-point increase in PASI score relative to week 33 [the time of withdrawal]). In total, 28% of the PASI 75 responders who were re-randomized to placebo at week 33 experienced a loss of adequate response compared to 5% of those who continued to receive adalimumab (P<.001).38 Of the patients who lost adequate response on re-randomization to placebo and then enrolled into the open-label extension trial, 38% (25/66) and 55% (36/66) regained a PASI 75 response after 12 and 24 weeks of retreatment, respectively.38 PASI 75 response rates for continuous and interrupted treatment were 84% and 45%, respectively, at week 52, 86% and 79% at week 76, and 75% and 73% at week 160 (LOCF).97 Retreatment was more effective in patients who restarted treatment before losing the PASI 50 response.
Etanercept is the drug for which there is the most experience in intermittent use and such regimens are indicated in the SPC. Although the efficacy of intermittent (as needed) treatment with etanercept is lower than that of the continuous treatment,98 in the CRYSTEL study 83% of patients regained the target clinical response (PGA≤2) with the first cycle of retreatment.99
In one study, patients receiving infliximab were re-randomized at week 14 to receive treatment every 8 weeks or as needed (infliximab was administered if the PASI 75 had been lost at the time of the visit corresponding to the infusion; if the response was maintained, placebo was administered).72 In the group of patients treated with a dose of 5mg/kg, the efficacy observed at week 50 in the patients on as needed treatment was lower than that of those who received continuous treatment every 8 weeks, in terms of both the PASI 75 (38.1% vs 54.5%) and PASI 90 (10.4% versus 34.3%) response rates. The percentage of patients in whom antidrug antibodies were detected was also higher in the intermittent regimen group (41.5% vs 35.8%) as was the rate of infusion reactions (9.2% vs 6.2%).72 Intermittent treatment (with the induction dose) is not recommended because of the unacceptable incidence of infusion reactions: 8 serious reactions (4%) occurred in the intermittent treatment group compared to 1 (<1%) in the group receiving continuous therapy, leading to the termination of the clinical trial.100
With respect to intermittent treatment with ustekinumab, in the PHOENIX 1 trial the median time to relapse after cessation of therapy (randomly assigned at week 40) was 15 weeks (27 weeks after the last dose), and 85% of the patients regained a PASI 75 response 12 weeks after restarting therapy, irrespective of dose.76 In the ACCEPT study, treatment of patients with a PGA score of 2 or less at week 12 was interrupted until the patient's condition was assessed as PGA 3 or higher. The median interval between these 2 events was 14.4 weeks in patients treated with ustekinumab 45 mg, 18.1 weeks in those treated with ustekinumab 90 mg, and 7.3 weeks in those treated with etanercept. Of the 633 patients retreated with ustekinumab after reaching a PGA of 3 or higher, 534 (84%) presented a PGA of 2 or less within 12 weeks of retreatment.54
According to the SPC for etanercept, there appears to be no correlation between the development of anti-etanercept antibodies and clinical response or adverse effects.37 There is evidence that the formation of antibodies against adalimumab, infliximab, and ustekinumab decreases serum drug concentrations and the response rate in patients with psoriasis.40,101,102 However, there is insufficient evidence to determine the usefulness of combination treatment with methotrexate or the measurement of drug levels or antidrug antibodies in the therapeutic management of patients with psoriasis. Combining the biologic with methotrexate is probably a useful way to extend drug survival. Data on drug levels and antibodies may be of use in making decisions regarding the replacement of the therapy (the presence of antibodies is an indication that the mechanism of resistance is immunologic and therefore specific to the drug).103
Combination Therapy With Other Systemic TreatmentsThe results of several clinical trials and retrospective studies in the literature indicate that the combination of biologic agents and conventional systemic treatments increases the effectiveness of treatment with no significant increase in adverse events.
Etanercept appears to be a particularly appropriate choice in such combined regimens104,105 and there is evidence supporting the usefulness of combinations of biologic agents with phototherapy106–110 methotrexate,111–113 and ciclosporin114 to increase efficacy, and with acitretin to achieve the same efficacy with a lower dose of etanercept.115
There is less published experience in the case of adalimumab, but favorable results have been reported for combinations with phototherapy, methotrexate, and other drugs.47,116,117
In the case of infliximab, the evidence is scant118,119 but the combination with methotrexate is a common practice used in over 50% of patients in some studies.120,121
Ustekinumab has been tested in a small series in combination with narrowband UV-B phototherapy to see whether the combination would accelerate and optimize response during the initial weeks of treatment.122
Patients being treated with any of the traditional systemic therapies who require biologic treatment because they have not achieved a PASI 50 response or have reached the cumulative toxic doses of their current treatment may need a transition period during which they receive a combination regimen of both drugs for a few weeks to prevent the relapse that might occur if systemic treatment were discontinued before the introduction of the biologic.123
For a more detailed explanation of the use of phototherapy, methotrexate, and acitretin in the treatment of psoriasis, see the relevant consensus statements124 and guidelines125,126 previously published by the AEDV Psoriasis Group.
ConclusionsThese guidelines represent a summary of the scientific evidence currently available on the efficacy of the biologic treatments indicated for psoriasis on the Spanish market at the time of publication and the selection criteria for the use of these drugs. The choice of biologic therapy must always be based on knowledge of the published response rates in clinical trials and take into account the course of the disease at the time of prescription and the characteristics of the patient, including age, sex, weight, comorbidities, and the presence or absence of active arthritis.
The most important factor in the choice of a biologic or in the calculation of the cost/benefit ratio for these drugs is the efficacy of the drug relative to baseline evaluated on the basis of scientific evidence from clinical trials. While the expected response in a population can be calculated from published PASI 50, PASI 75, and PASI 90 response rates, this information does not allow us to predict the response of individual patients.
The outcome of any therapeutic intervention must be assessed within a maximum of 3 months. In most patients and for all of the biologic agents used to treat psoriasis, maximal therapeutic efficacy is achieved within a maximum period of 6 months.
According to a recent meta-analysis, the incremental efficacy (risk difference vs placebo) in terms of achieving a PASI 75 response at 24 weeks for the different biologic agents is as follows: infliximab 78% (95% CI, 72-83); ustekinumab 90 mg, 77% (95% CI, 71%-83%); ustekinumab 45 mg, 70% (95% CI, 64%-77%); adalimumab 60% (95% CI, 45%-74%); etanercept 100 mg/wk for 12 weeks followed by 50 mg/wk, 50% (95% CI, 42%-57%); and etanercept 50mg/wk, 45% (95% CI, 34%-56%).31
In the choice of initial treatment, the dermatologist should take into account the likelihood of primary treatment failure, the appropriateness of the frequency and route of administration, as well as other issues that fall outside the scope of these guidelines, such as the economic impact of the decision (cost of the drugs required for both the induction and maintenance phases).
Table 7 summarizes other aspects that should be considered when prescribing biologics, such as pharmacokinetics, route of administration, safety, as well as their indication in children and in patients with psoriatic arthritis.
Because biologic agents are immunogenic, the patient may produce antibodies against the drug. The immune response depends on the drug used and correlates with reduced adherence to treatment, loss of response, and—in the case of infliximab—an increased risk of infusion reactions which makes intermittent treatment unadvisable.
The strategies that can be used in the case of primary or secondary treatment failure with a biologic agent include combination therapy with a systemic agent, phototherapy, photochemotherapy, or temporary intensification of the biologic therapy; however, in general, the preferred strategy is to switch the patient to another biologic agent.
The combination of biologic agents with conventional systemic treatments increases efficacy with no significant increase in adverse events.
Final CommentsThese guidelines do not represent an exhaustive review of the literature or the SPCs for the drugs discussed, but rather focus on the practical aspects of treatment and the selection of an appropriate biologic agent. The prescribing physician should carefully read the instructions in each SPC and compare them with the recommendations in this consensus statement, particularly with regard to dose, contraindications, and possible interactions. The authors would appreciate feedback from readers about any inaccuracies and would welcome help in the task of updating the guidelines as additional information becomes available.
Ethical DisclosuresProtection of human and animal subjectsThe authors state that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace concerning the publication of patient data, and that all the patients included in this study have been appropriately informed and gave their written informed consent to participate in this study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors have participated in clinical trials, have served as consultants, and/or received speaker's fees or funding to attend training events from Abbvie (formerly Abbott), Janssen, MSD, and Pfizer.
Please cite this article as: Puig L, et al. Directrices españolas basadas en la evidencia para el tratamiento de la psoriasis con agentes biológicos, 2013. I. Consideraciones de eficacia y selección del tratamiento. Actas Dermosifiliogr. 2013;104:694–709.