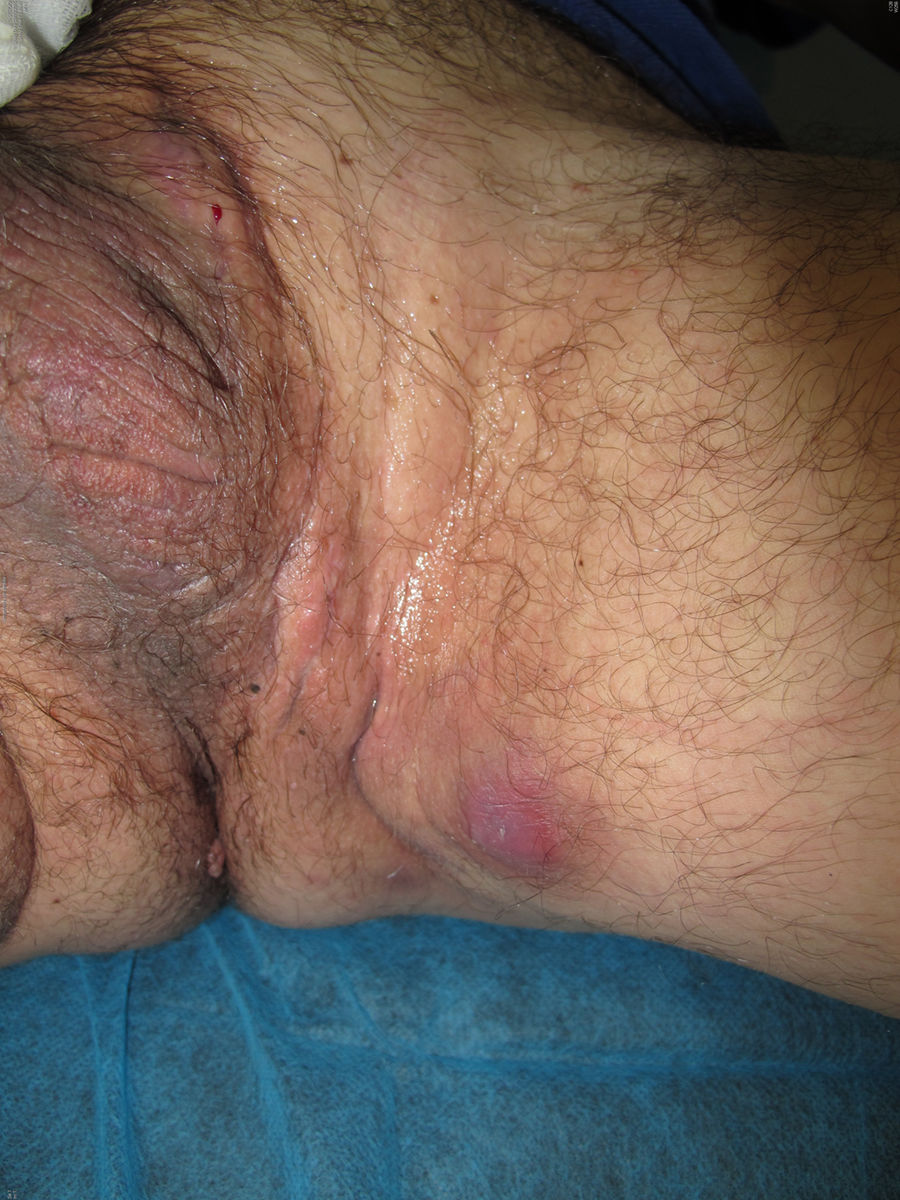
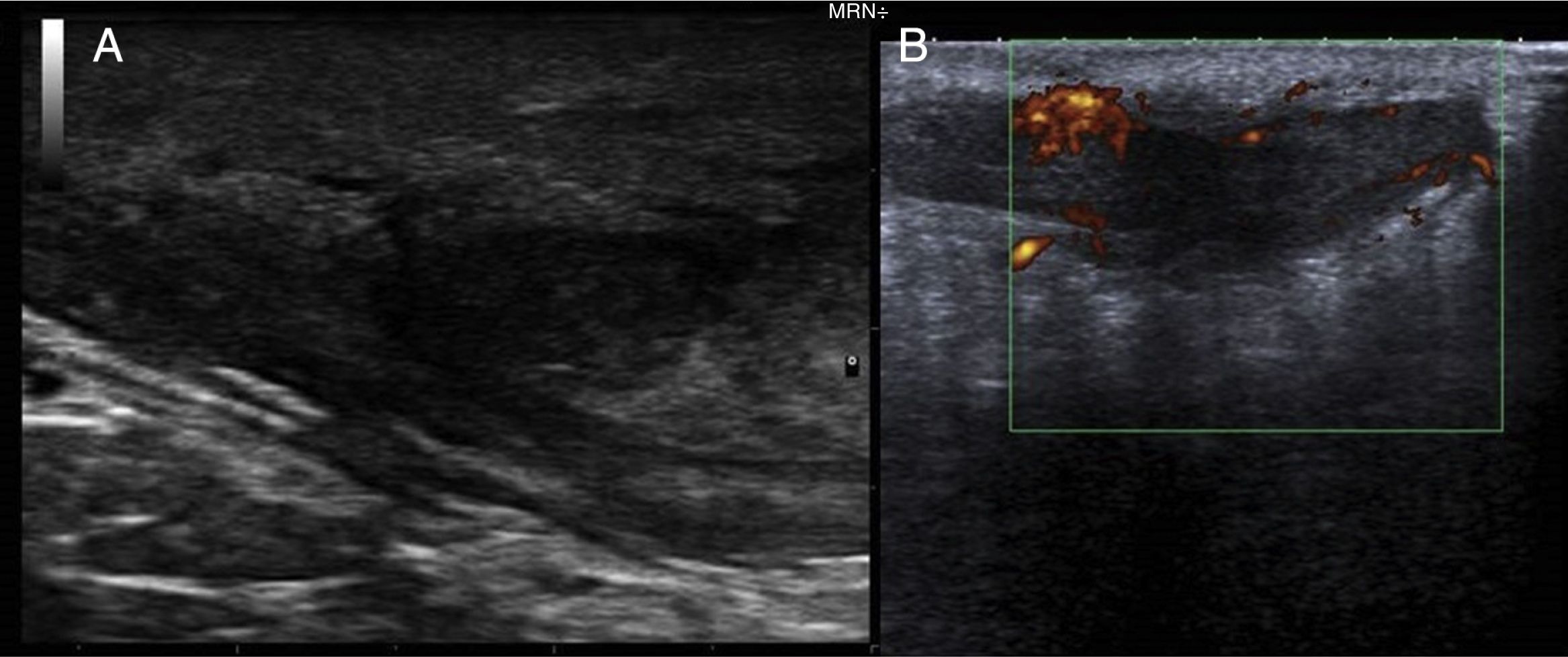
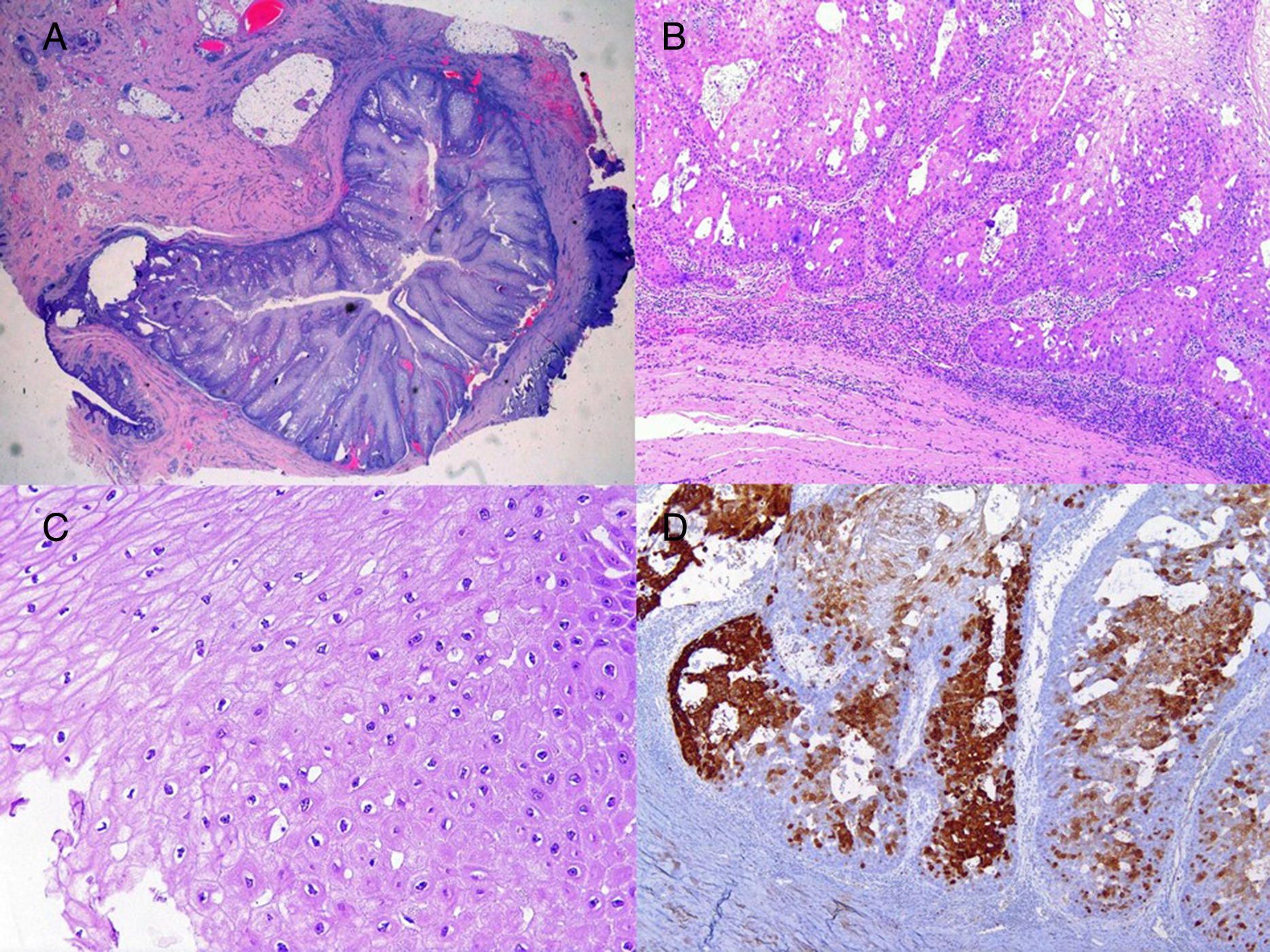
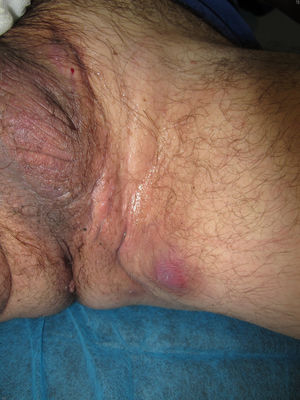
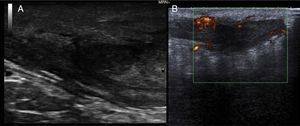
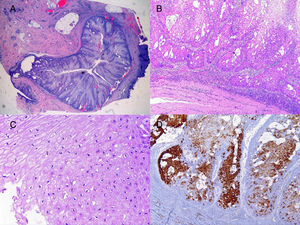
We report the case of a 51-year-old man who was referred to the dermatology department with a painful, suppurative nodule in his left groin that had appeared 1 year previously and was refractory to various antibiotic regimens prescribed by his primary care physician. The patient had a personal history of type 2 diabetes mellitus and was a smoker. Physical examination revealed an erythematous subcutaneous lesion with areas of suppuration in the lower left groin. On palpation, the lesion extended to the perineal region (Fig. 1). The examination also revealed 3 lesions that were clinically compatible with condylomata acuminata in the perianal area. He was diagnosed clinically for hidradenitis suppurativa (HS). Ultrasonography with an 18-MHz linear probe revealed the presence of a fistulous tract, with areas of fluid collection, together with intense vascularization on Doppler mode (Fig. 2). Given the location and depth of the lesion, the patient was sent to the clinic of the surgery department for complete removal of the lesion. Histopathology showed changes that were compatible with HS and well-differentiated squamous cell carcinoma (SCC) over the fistulous tract, which was located close to the deep resection margin. No vascular invasion or perineural infiltration was observed (Fig. 3 A and B). Extension of the surgical margins revealed no evidence of residual tumor, although foci suggestive of viral infection were observed (Fig. 3C). Immunohistochemical staining with p16 was intensely positive (Fig. 3D). Polymerase chain reaction and genotyping based on in situ hybridization with a microarray of paraffin-embedded tissue were performed to investigate the presence of human papillomavirus (HPV). The result was positive for genotype 6 (considered low risk); the same finding was recorded for the perianal condylomata acuminata. Laboratory analysis yielded negative results for HIV and hepatotropic viruses. After a 12-month clinical and imaging-based follow-up (computed tomography and magnetic resonance imaging of the pelvis), the patient showed no signs of local recurrence or metastasis.
During the last few decades, there have been reports of cases of carcinomatous transformation in HS in the perineal, perianal, and gluteal region. Of particular interest is the review of Lagoviez et al.,1 who report 13 new cases and investigate the presence of HPV in 8 histology samples. According to the literature, the condition predominantly affects men. This observation is logical, given that the locations of HS reported above are typical in men.2
A key question in this disease at present is whether malignant degeneration occurs more frequently in the perianal or gluteal region owing to HPV infection in these areas. The virus seems to be implicated in the pathogenesis of SCC through oncogenic expression.3 This has also been reported in the development of other types of intraepithelial neoplasia and tumors in the anogenital region, affecting the penis, scrotum, and anal, vulvar, and vaginal areas.4,5 Twenty years ago, Li et al.6 first put forward the hypothesis that HPV played an important role in the SCC that arises in HS, although they did not perform a histopathology analysis.6 However, in a later study, Lagoviez et al.1 investigated the presence of HPV in 8 anogenital tumors using polymerase chain reaction. The results were positive in all 8 samples, both for the low-risk genotypes (HPV-6) and for the high-risk genotypes (HPV-16 and 68). It was recently demonstrated that even HPV genotypes that are low-risk may be involved per se in the development of anogenital SCC.7
Flores et al.8 observed a significant positive correlation in HPV-16 viral load between nearby sites of infection, such as the anal canal and the perianal area or the perianal area and the scrotum, or sites that are in regular contact in a resting position. Therefore, previously uninfected genital areas could become inoculated by infections at nearby anatomical sites through direct contact and facilitated by environmental factors such as humidity, temperature, and, possibly, poor hygiene.
Furthermore, the advent of anti–tumor necrosis factor therapy in HS has led various authors to recommend investigating the presence of lesions suggestive of HPV in these areas, since there have even been reports of deaths resulting from the development of SCC after initiation of this therapy.1,9
Both ultrasound and magnetic resonance imaging could prove useful for confirmation of the true anatomical extension of the disease before surgery.10 Wide excision of the lesion is considered the standard treatment. SCC that arises in areas of chronic inflammation caused by HS is often well differentiated. Nevertheless, despite this theoretically good histologic prognosis, we can observe rapid growth, local aggressiveness, early metastasis, and high mortality.1
The case we report supports the relevant role of HPV in the development of SCC in patients with HS in the inguinal, perineal, and perianal areas.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Segura Palacios JM, García Montero P, Fúnez Liébana R, Repiso Jiménez JB. Human Papilloma Virus and the Risk of Squamous Cell Carcinoma Arising in Hidradenitis Suppurativa. Actas Dermosifiliogr. 2018;109:457–459.