Topical minoxidil solution (TMS) has been used since 1986 to treat androgenetic alopecia.1 Frequent side effects include local intolerance and itching.2 Hypertrichosis has also been observed close to the areas where the solution is applied, mainly the face and especially the temple and the preauricular region.2–4 In contrast, generalized or distant hypertrichosis is an uncommon side effect of TMS, and its etiology and pathogenesis remain unknown.
We report the case of a 42-year-old woman diagnosed with androgenetic alopecia who visited our clinic with generalized new hair growth after using 5% TMS for 2 weeks. The patient had been treated with a compounded formulation (5% minoxidil propylene glycol, 0.5% progesterone, and 0.05% clobetasol), which she had been applying twice daily. She remembered having used a random amount and carefully avoiding contact between the product and regions outside the scalp.
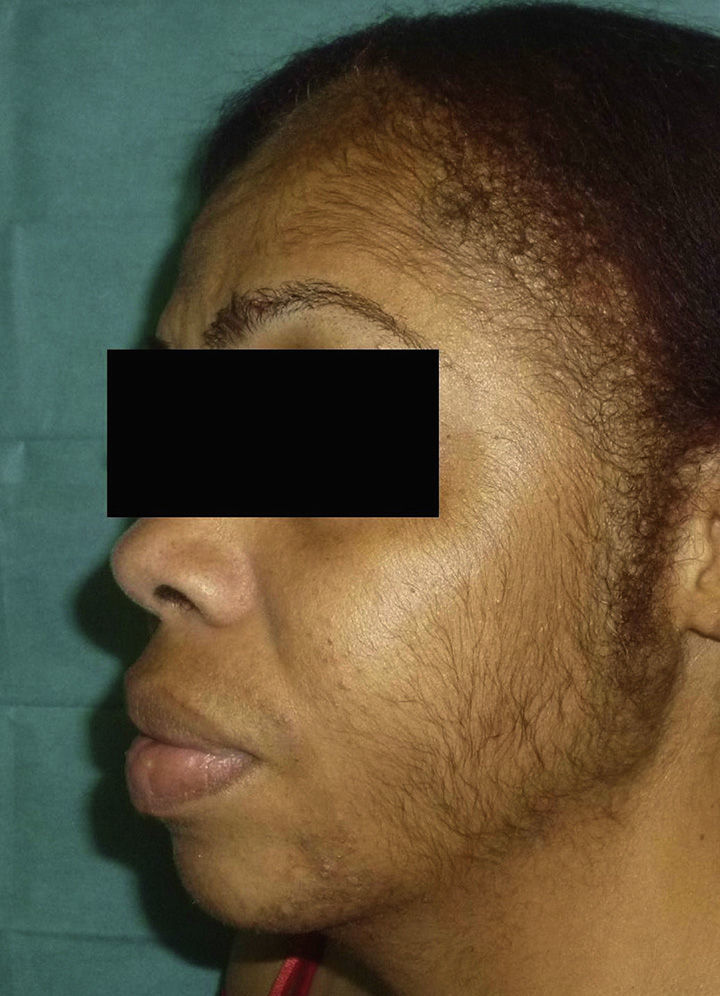
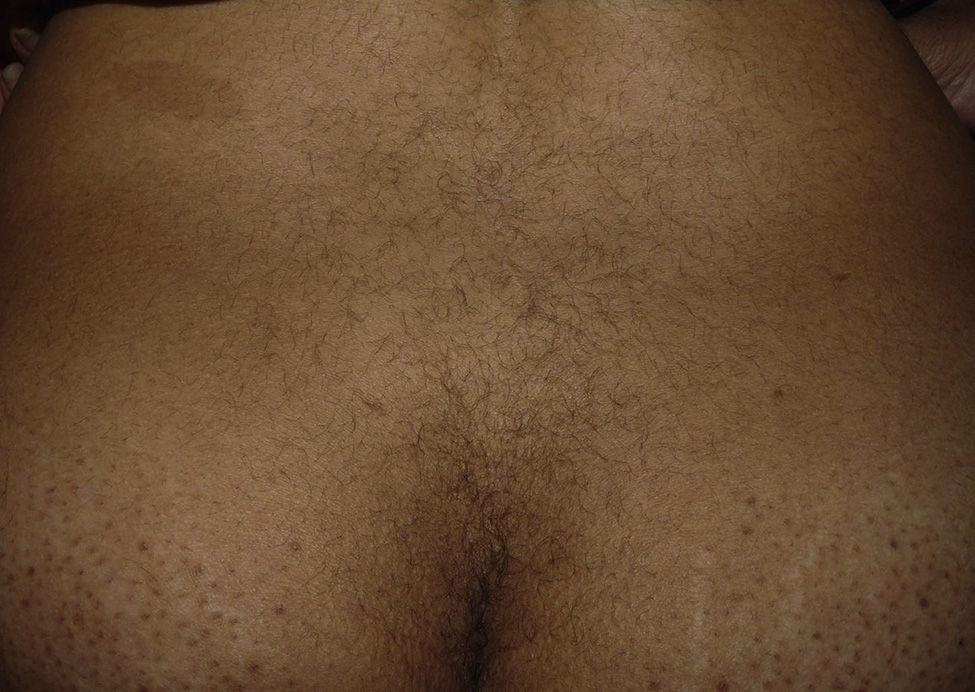
The patient reported having a certain amount of vellus facial hair before starting treatment. However, after 2 weeks of treatment, she noticed new growth of body hair, which became darker and thicker. The main regions affected were the forehead, cheeks, beard area, chest, lower back, arms, and legs (Figures 1 and 2). Hormonal tests (follicle-stimulating hormone, prolactin, testosterone, dehydroepiandrosterone sulfate, and δ-4-androstenedione) revealed no abnormalities.
Six months after stopping TMS, the patient's condition began to improve, with persistence of hair on the beard area and trunk. Ten months after stopping treatment, the improvement has persisted, although her condition has not returned to normal.
Between 0% and 5% of patients who receive TMS are affected by hypertrichosis—mainly on the face—caused by local transfer of the product.2,3
Dawber and Rundegren3 studied facial hair growth and hypertrichosis (defined as new hair growth on parts of the body other than the face) as separate entities in women using 5% and 2% TMS compared with placebo. They observed more cases of hypertrichosis in the group treated with 5% TMS (up to 2% of cases after use). They also observed that the adverse effect was more common in women than in men and that within this group, the effect was more common in women aged >50 years and women with facial hair before starting treatment. The facial hair was often vellus hair. Of note, there is a clear association between androgenetic alopecia and hyperandrogenism, which leads to the onset of symptoms such as hirsutism,3,5 one of the main risk factors for development of hypertrichosis after treatment with TMS. Furthermore, individuals of Mediterranean, Hispanic, and Middle Eastern ethnicity more often have facial hair.
In the cases reported in the literature, hypertrichosis usually appears 2 to 3 months after starting treatment and disappears 1 to 5 months after discontinuation.4,6 As for the present case, the effect was earlier and more persistent. Although we cannot rule out synergistic action of topical clobetasol, the literature on hypertrichosis caused by topical corticosteroids shows that hypertrichosis appears after longer periods of use and is limited to the treatment site7 or neighboring sites.8 No effects at distant sites have been reported.
Several hypotheses have been put forward to account for TMS-induced hypertrichosis.3 The initial recommendation was to apply the product manually or using an applicator, although these hypotheses do not explain the finding of generalized hypertrichosis.
Despite the dose-dependent onset of this side effect, which is more common in 5% TMS than in 2% TMS and when the amount applied exceeds the recommended dose,2,3 minoxidil was not detected in blood in any of the cases reported. Therefore, we can deduce that the response is idiosyncratic and that it results from hypersensitivity of the follicles to the product, leading to the onset of hypertrichosis, even at distant sites, in more sensitive patients.
Given that excess facial hair before treatment implies an increased risk of developing TMS-induced hypertrichosis,3 we must treat such cases with a lower concentration of minoxidil or choose an alternative therapy.
In conclusion, before prescribing TMS, physicians should take a full clinical history and perform a physical examination to establish the risk of developing hypertrichosis, which would be highest in middle-aged women with facial hair at diagnosis. In addition, every effort should be made to avoid combination with other products whose synergistic action is not well-studied and, therefore, whose effect is not predictable, as is the case with topical corticosteroids.
Please cite this article as: Gargallo V, Gutierrez C, Vanaclocha F, Guerra-Tapia A. Hipertricosis generalizada secundaria a minoxidil tópico. Actas Dermosifiliogr. 2015;106:599–600.