Paracoccidioidomycosis (PCM) is a systemic mycosis caused by a dimorphic fungus.
The fungus Paracoccidioides brasiliensis lives in the ground and on plants in tropical and subtropical regions. It is endemic to South America and is the most common of the deep mycoses in Paraguay.
Infection occurs by the inhalation of spores during the first decades of life; these spores reach the lungs and can spread to other parts of the body via the blood or lymphatics. The fungus can affect the skin, mucosas, adrenal glands, and other organs.1–4
The 2 main clinical presentations of PCM are the chronic form and the acute/subacute form. The chronic form, which accounts for up to 90% of cases, typically affects male farmers between 30 and 60 years of age. The higher frequency in men is due to the presence of 17-β-estradiol receptors in the cytoplasm of P. brasiliensis. The interaction between the receptors and the female hormone inhibits transformation of the fungus from the mycelial form to the yeast form; this transformation is essential for infection to develop.3–7
The acute/subacute form (juvenile type) accounts for less than 10% of cases and is mainly observed in young individuals with a depression of cell-mediated immunity. This form has a rapid clinical course (4 to 12 weeks), with alterations of the monocyte-macrophage system and polymorphous skin lesions (nodular, furunculoid, verrucous, ulcer-granulomatous, molluscoid).5,7–9
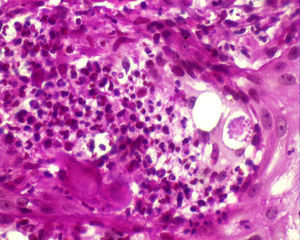
The gold standard for diagnosis is culture, which must be performed under strict biosafety measures. The fungus grows as mycelia at room temperature and as the yeast in vitro and in tissues at 37°C.1-5 Other diagnostic methods include serology (immunodiffusion), and molecular studies such as polymerase chain reaction.1,2,10 Histopathology reveals fungal structures, with well or poorly organized granulomas, depending on the immune response.1,7
Commonly associated infectious diseases include tuberculosis (5%-10%), intestinal parasitoses, Chagas disease, syphilis, other superficial and deep mycoses, and AIDS. Important noninfectious diseases that may be associated with PCM are non-Hodgkin lymphoma and some carcinomas.
An association with smoking has been reported in a high percentage of patients.1,5,8
Patients with severe forms of PCM, with weight loss of over 10%, respiratory difficulty, and neurological symptoms, must be hospitalized.
The treatment of choice is itraconazole, 200-400mg/d, by mouth for 6 to 12 months; this regimen achieves cure in 88% to 100% of cases, though there is a recurrence rate of 3%. In severe forms, amphotericin B, 0.8-1mg/kg/d, is administered by intravenous infusion until an improvement is achieved in the clinical manifestations, after which the patient can be changed to oral treatment.1–3,7 Other alternatives include trimethoprim-sulfamethoxazole, voriconazole, and fluconazole. Periodic follow-up of clinical, mycologic, radiologic, and immunologic criteria must be performed to determine whether a favorable response to treatment is being achieved. Delays in the initiation of treatment can elevate mortality to up to 30%, and increase the risk of potentially disabling sequelae, such as pulmonary fibrosis.1–3,5,7
We present the case of a 21-year-old housewife, an indigenous woman from a humid and wooded rural region of Paraguay. She had a 2-month history of skin lesions on her back, face, neck, abdomen, and limbs. The patient described a feeling of fever, mainly in the evening, jaundice, dyspnea, asthenia, anorexia, and unquantified weight loss. She denied any underlying diseases, smoking, alcohol consumption, or use of recreational drugs.
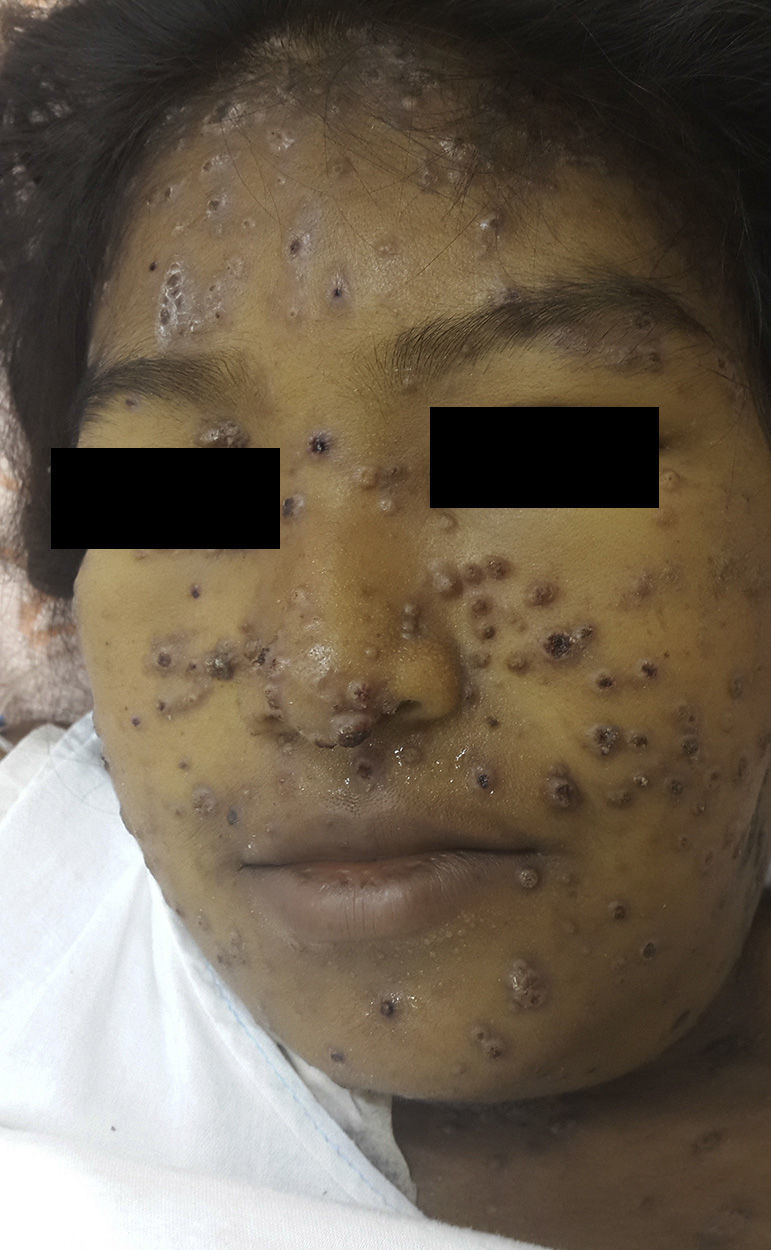
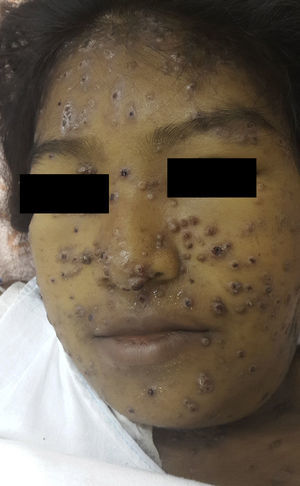
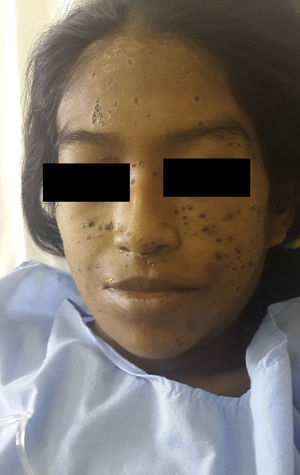
Physical examination revealed erythematous-violaceous papules and plaques, some with a central depression and bloodstained scab, mainly on the face (Fig. 1). In addition, jaundice, ascites, and submandibular and cervical lymph nodes of 1.5 to 3cm diameter were detected. Breath sounds were absent in both lung bases. The patient presented fever of 38.5°C, but her vital signs were stable.
Blood test results were as follows: hemoglobin, 7.4g/dL; hematocrit, 21%; white cell count, 11800/μL (neutrophils, 82%; lymphocytes, 10%; monocytes, 6%; and eosinophils, 2%); platelets, 124x103/μL; aspartate aminotransferase, 127U/L (normal value, 32U/L); alanine aminotransferase 75U/L (normal value, 33U/L); alkaline phosphatase, 4694IU/L (normal value, 300 IU/L); total bilirubin, 10.1mg/dL; direct bilirubin, 4.8mg/dL; indirect bilirubin, 6.1mg/dL; albumin, 1.3g/dL (normal value, >3.5g/dL); prothrombin index, 50%; activated partial thromboplastin time, 45seconds. Serology for human immunodeficiency virus, syphilis, hepatitis C virus, hepatitis B surface antigen, and hepatitis A virus was negative. Cultures of sputum and gastric fluid were negative for acid- and alkali-fast bacilli and for fungi.
Abdominal ultrasound revealed hepatomegaly and a large volume of free fluid in the abdominal cavity; there was a bilateral pleural effusion. Abdominal computed tomography showed no involvement of the adrenal glands.
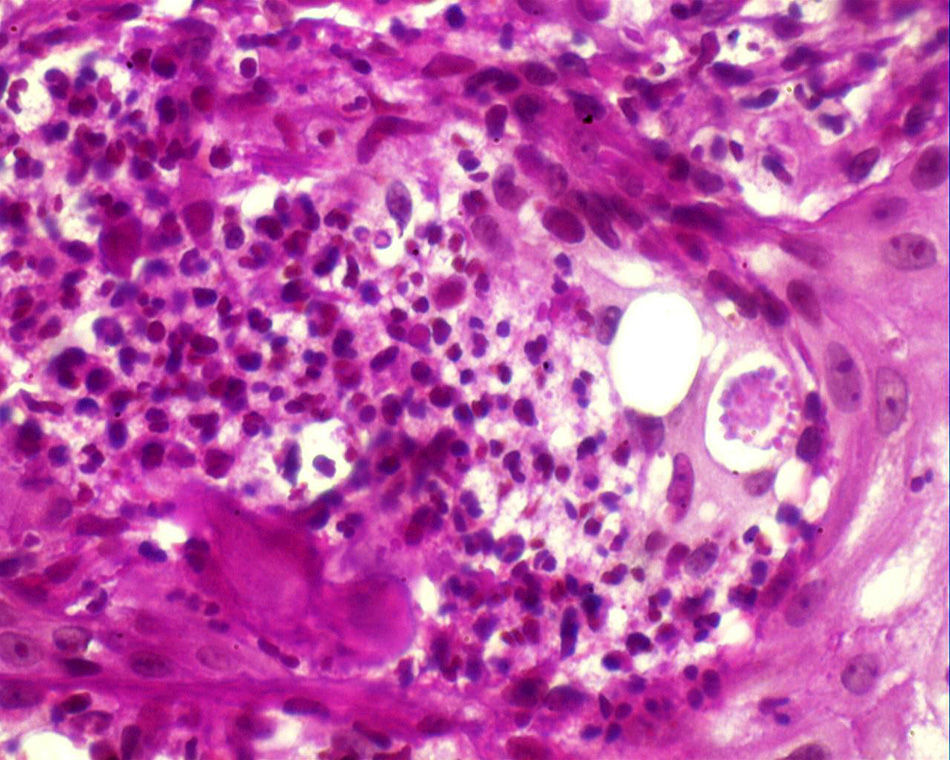
Histopathology confirmed the presence of pseudoepitheliomatous hyperplasia and pandermal suppurative granulomatosis, with multinucleated giant cells and mycotic spores with birefringent cell walls and exosporulation (Fig. 2).
A smear from a lymph node revealed multinucleated giant cells with mycotic spores compatible with P. brasiliensis. Based on this information, we made a final diagnosis of acute disseminated PCM.
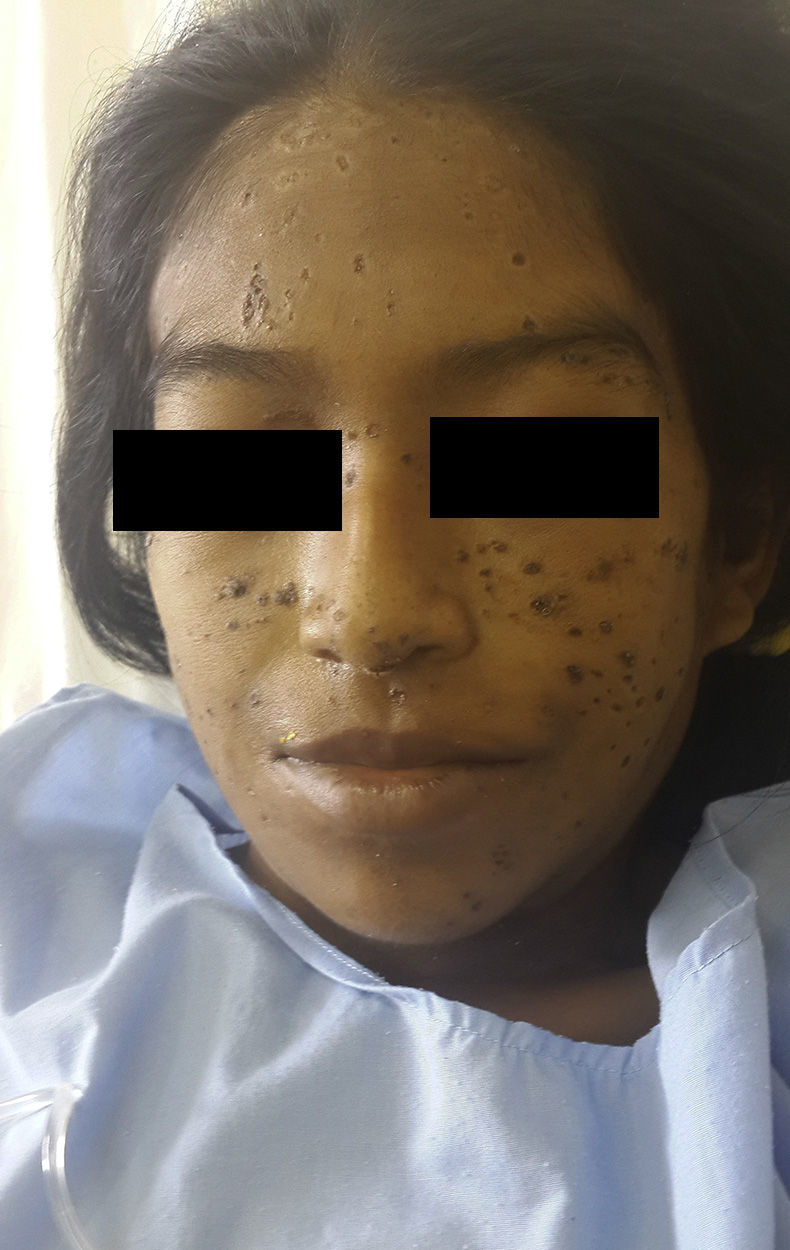
Treatment was prescribed with 3g of amphotericinB at a dose of 50mg/d (although administration had to be interrupted for several days due to transitory alterations of kidney function), leading to an improvement in the skin lesions (Fig. 3), liver function (alkaline phosphatase, 1256IU/L after 1 month of treatment), and in the patient's general state. Treatment was completed with itraconazole, 200mg/d, for 6 months after discharge. At the time of writing, the patient has been on treatment for 2 months, with a cumulative dose of amphotericin of 2.5g. There has been no recurrence.
PCM is characterized by marked polymorphism of the skin lesions, which present as papules, nodules, desquamating erythematous verrucous plaques, and ulcers with a granulomatous base and petechiae; the molluscoid lesions seen in our patient, mainly affecting the face in the area of the nose and mouth, are very rare.1
This type of molluscoid lesion obliges us to rule out cryptococcosis, histoplasmosis, atypical mycobacteria, molluscum contagiosum, disseminated leishmaniasis, and leprosy.1,2,5,7
We draw attention to the fact that the patient was a young woman of childbearing age who came from an endemic area with favorable environmental conditions for the fungus. She did not present immunosuppression except for malnutrition. Molluscoid lesions were visible mainly on the face, requiring us to rule out a long list of other diagnoses and comorbid conditions. Migratory phenomena make it necessary for dermatologists to be aware of infectious diseases that can arise in other continents.10
Please cite this article as: Di Martino Ortiz B, Moreno T, Galeano G, Rodríguez M. Paracoccidioidomicosis aguda diseminada moluscoide en mujer joven. Actas Dermosifiliogr. 2015;106:597–599.