Imiquimod cream is a topical imidazoquinoline amine widely used in dermatology. It has antitumor, antiviral, and immunomodulatory properties that modify biologic response.1 It was first approved in 1997 for the treatment of genital warts and later received authorization for use in superficial basal cell carcinoma and actinic keratosis. Treatment regimens vary according to the disease. The summary of product characteristics for imiquimod mentions the possibility of “mild or moderate hypopigmentation” following its use2 but total loss of pigmentation, which is particularly relevant in the genital region, is not mentioned as a possible adverse effect.
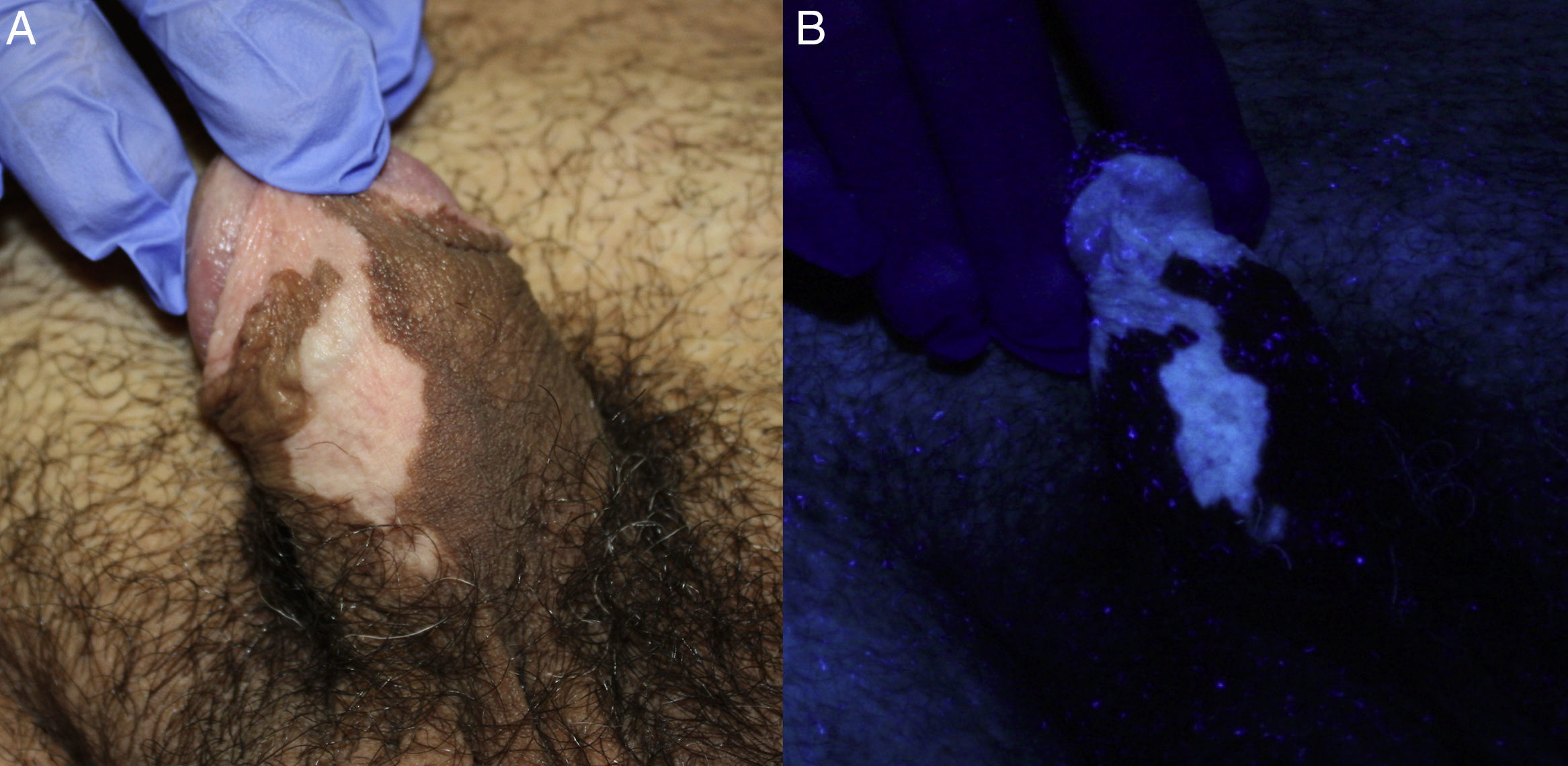
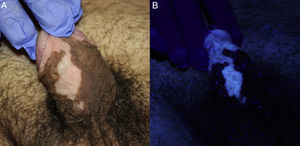
Case DescriptionsA 45-year-old man with Fitzpatrick skin type iv and no relevant personal medical history presented with genital warts of 1 year duration. After screening for other sexually transmitted infections (STIs), the affected area was treated with cryotherapy. Two months later, the patient was administered another cycle of cryotherapy as the lesions had not cleared. He was also informed about the possibility of treatment with 5% imiquimod 3 times a week on alternate days if the warts did not improve. The lesions persisted and the patient was treated with 5% imiquimod for 16 weeks. At the next follow-up visit, the warts were found to have cleared completely, but there were several achromic macules on the shaft of the penis and foreskin that were more evident when viewed under Wood's light (Fig. 1). The rest of the physical examination was unremarkable. A blood workup including thyroid hormones, antithyroid antibodies, and antinuclear antibodies showed results within normal ranges. The lesions have remained stable without treatment over 4 years of follow-up and there has been no loss of pigmentation in any other areas.
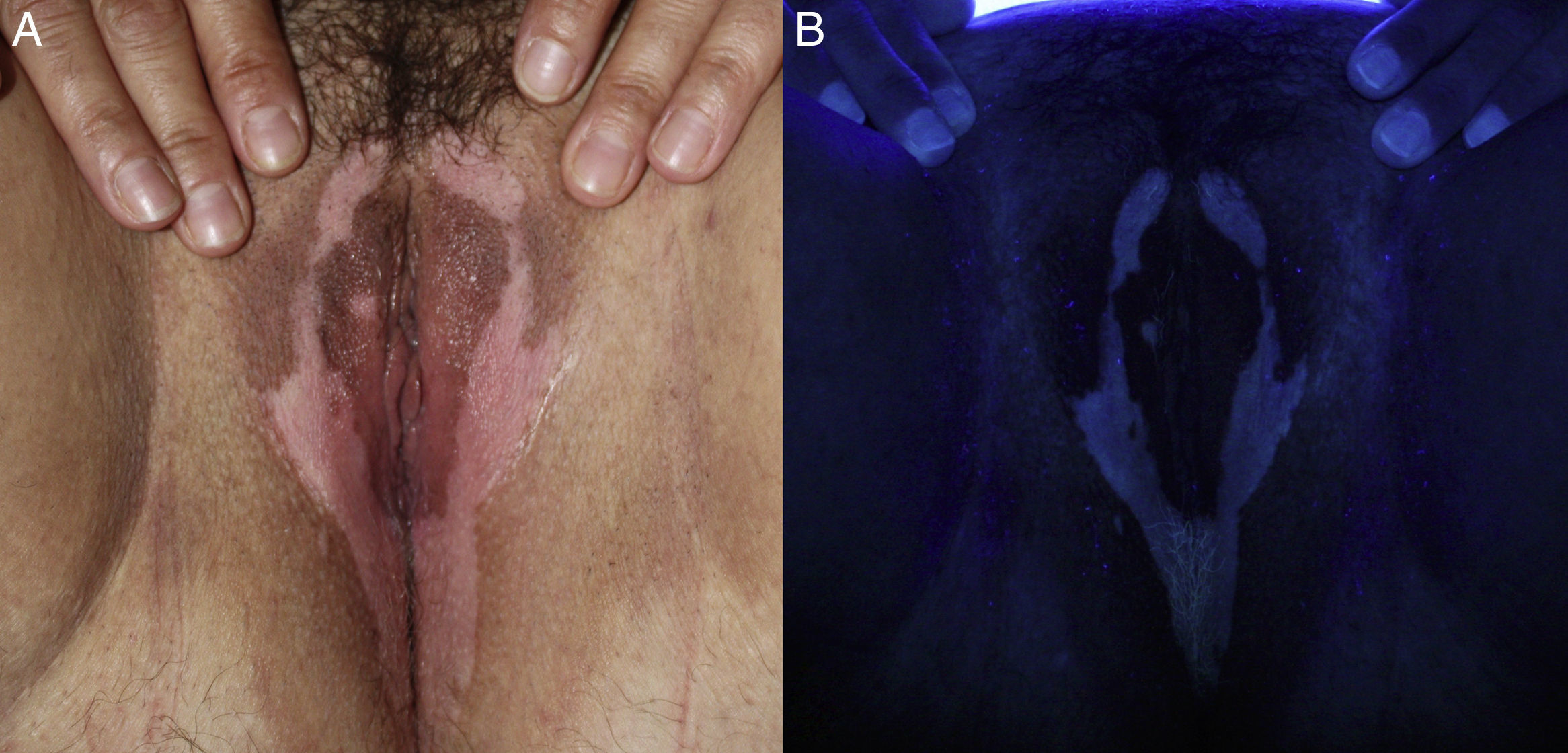
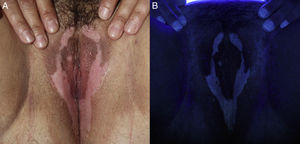
A 41-year-old woman with Fitzpatrick skin type iv and no relevant personal history presented with vulvar and perineal warts that had been present for 1 month. The patient was tested for other STIs and given a cervical smear and prescribed 5% imiquimod 3 times a week (on alternate days). At the next follow-up visit, after 16 weeks of treatment with imiquimod, the warts had disappeared completely. The genital examination revealed the presence of milky white symmetric stains, which were more pronounced under Wood's light, along the labia majora and perineum (Fig. 2). The rest of the physical examination was unremarkable. A blood workup including thyroid hormones, antithyroid antibodies, and antinuclear antibodies showed no alterations. The lesions have remained stable without treatment for 4 months and there has been no pigment loss in any other areas.
We have described 2 cases of vitiligo-like pigmentation secondary to the use of 5% imiquimod. When asked, both patients indicated that this loss of pigmentation would interfere with their sexual relations. We proposed performing a histologic examination but the patients refused.
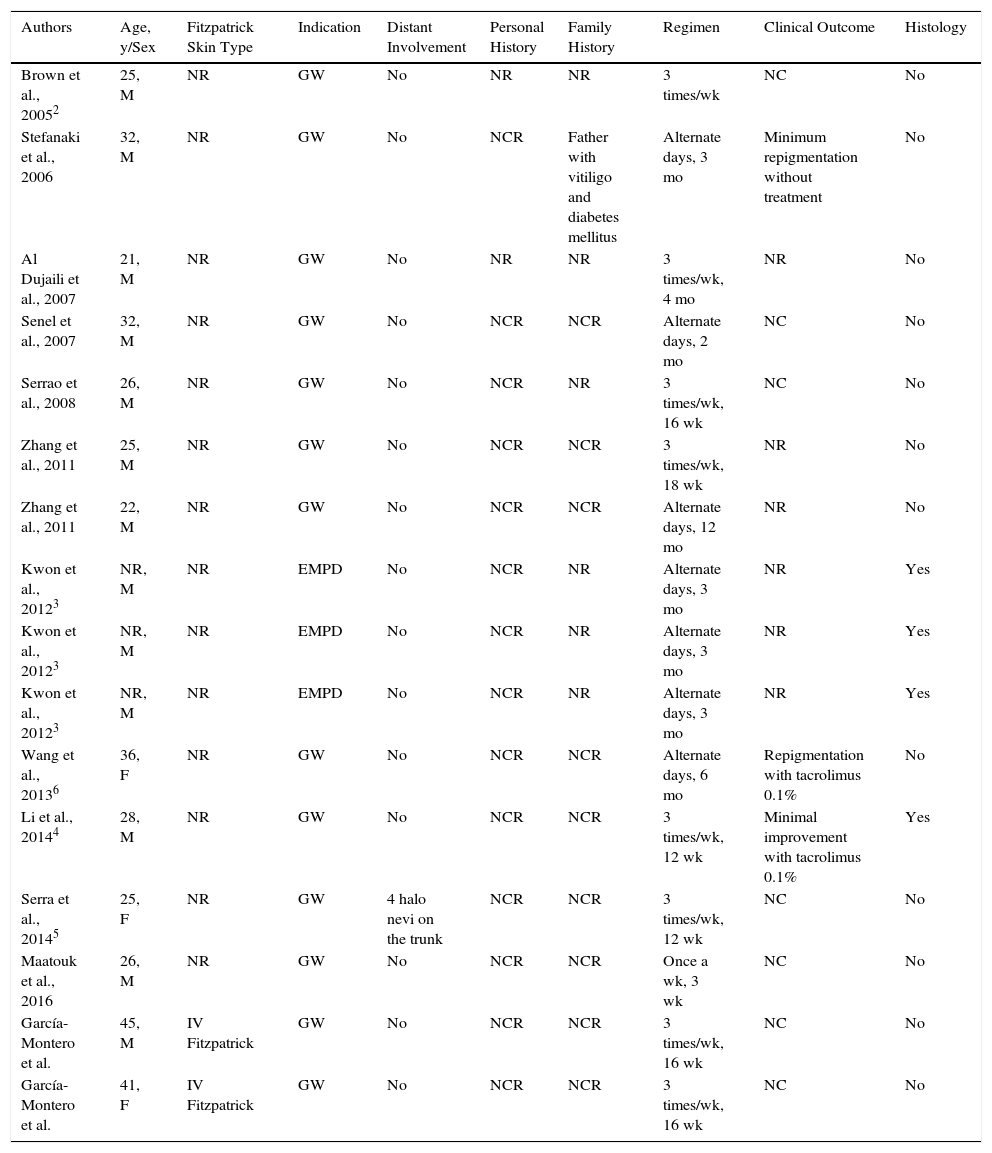
DiscussionFourteen cases of vitiligo-like hypopigmentation in the genital area have been described following the use of 5% imiquimod2–6 (Table 1). The cream was used to treat genital warts in 11 cases and extramammary Paget disease in three.3 Treatment duration ranged between 3 and 18 weeks. Histologic examination was performed in 4 cases and showed similar findings to those seen in vitiligo.3,4 One of the patients developed multiple concomitant halo nevi on the trunk.5 Tacrolimus 0.1% ointment resulted in a slight improvement in 2 patients,4,6 but repigmentation did not occur in the vast majority of cases over a maximum follow-up period of 18 months. Pronounced hypopigmentation in the genital area has not been described in association with other techniques, such as cryotherapy, curettage, or carbon dioxide laser therapy, but there have been reports of vitiligo-like extragenital lesions in basal cell carcinomas treated with 5% imiquimod.
Summary of Cases of Vitiligo-Like Hypopigmentation Secondary to 5% Imiquimod.
| Authors | Age, y/Sex | Fitzpatrick Skin Type | Indication | Distant Involvement | Personal History | Family History | Regimen | Clinical Outcome | Histology |
|---|---|---|---|---|---|---|---|---|---|
| Brown et al., 20052 | 25, M | NR | GW | No | NR | NR | 3 times/wk | NC | No |
| Stefanaki et al., 2006 | 32, M | NR | GW | No | NCR | Father with vitiligo and diabetes mellitus | Alternate days, 3 mo | Minimum repigmentation without treatment | No |
| Al Dujaili et al., 2007 | 21, M | NR | GW | No | NR | NR | 3 times/wk, 4 mo | NR | No |
| Senel et al., 2007 | 32, M | NR | GW | No | NCR | NCR | Alternate days, 2 mo | NC | No |
| Serrao et al., 2008 | 26, M | NR | GW | No | NCR | NR | 3 times/wk, 16 wk | NC | No |
| Zhang et al., 2011 | 25, M | NR | GW | No | NCR | NCR | 3 times/wk, 18 wk | NR | No |
| Zhang et al., 2011 | 22, M | NR | GW | No | NCR | NCR | Alternate days, 12 mo | NR | No |
| Kwon et al., 20123 | NR, M | NR | EMPD | No | NCR | NR | Alternate days, 3 mo | NR | Yes |
| Kwon et al., 20123 | NR, M | NR | EMPD | No | NCR | NR | Alternate days, 3 mo | NR | Yes |
| Kwon et al., 20123 | NR, M | NR | EMPD | No | NCR | NR | Alternate days, 3 mo | NR | Yes |
| Wang et al., 20136 | 36, F | NR | GW | No | NCR | NCR | Alternate days, 6 mo | Repigmentation with tacrolimus 0.1% | No |
| Li et al., 20144 | 28, M | NR | GW | No | NCR | NCR | 3 times/wk, 12 wk | Minimal improvement with tacrolimus 0.1% | Yes |
| Serra et al., 20145 | 25, F | NR | GW | 4 halo nevi on the trunk | NCR | NCR | 3 times/wk, 12 wk | NC | No |
| Maatouk et al., 2016 | 26, M | NR | GW | No | NCR | NCR | Once a wk, 3 wk | NC | No |
| García-Montero et al. | 45, M | IV Fitzpatrick | GW | No | NCR | NCR | 3 times/wk, 16 wk | NC | No |
| García-Montero et al. | 41, F | IV Fitzpatrick | GW | No | NCR | NCR | 3 times/wk, 16 wk | NC | No |
Abbreviations: EMPD, extramammary Paget disease; F, female; GW, genital warts; M, male; NC, no change; NCR, not clinically relevant; NR, not reported.
Several theories have been proposed to explain imiquimod-induced vitiligo-like hypopigmentation. First, imiquimod might directly induce apoptosis of melanocytes7; second, it might stimulate toll-like receptor 7, triggering the release of diverse cytokines, which would activate T cells, causing the destruction of melanocytes8; and finally pigment loss could be due to the Koebner phenomenon in predisposed patients.9 Regardless of the mechanism of this adverse effect, hypopigmentation in the genital area can have considerable emotional and sexual repercussions. Several studies have shown that vitiligo has an impact on a person's sex life, and genital involvement has been statistically associated with worsening of sexual relations.10
It is therefore essential to inform patients of the risk of hypopigmentation with 5% imiquimod and to stop treatment when pigment loss begins. Although vitiligo-like hypopigmentation is very rare in this setting, it would seem wise to identify at-risk patients (i.e., patients with dark skin, a personal or family history of vitiligo, halo nevi or postinflammatory hypopigmentation) in order to prevent its occurrence.
Please cite this article as: García-Montero P, Jiménez JBR, Morano MTF, Martín MdT. Hipopigmentación genital similar a vitíligo tras tratamiento con imiquimod 5%. Actas Dermosifiliogr. 2017;108:378–380.