Biologic agents are now being used to treat chronic inflammatory diseases, mainly those of rheumatic, dermatologic, or gastrointestinal origin. This development has led to a reassessment of the diagnosis and therapeutic management of their associated adverse effects, which can include relapses of certain pre-existing diseases that a patient may have; although these relapses have been successfully treated in some cases, they have been difficult to control in others.1 We present the case of a patient with infliximab-treated ulcerative colitis who developed a relapse of atopic dermatitis, and we review the cases reported in the literature.
The patient was a 30-year-old man with a past history of ulcerative colitis on treatment with infliximab; the disease had been refractory to treatment with mesalazine and azathioprine. After the fifth infusion he developed widespread, intensely pruritic eczema that did not respond to topical corticosteroids or oral antihistamines.
The patient had a history of atopic dermatitis (AD) since childhood, but denied rhinitis or extrinsic asthma. The AD lesions had always developed in flexures (cubital and popliteal fossae) and had responded to corticosteroids and topical calcineurin inhibitors, without requiring systemic treatment or phototherapy.
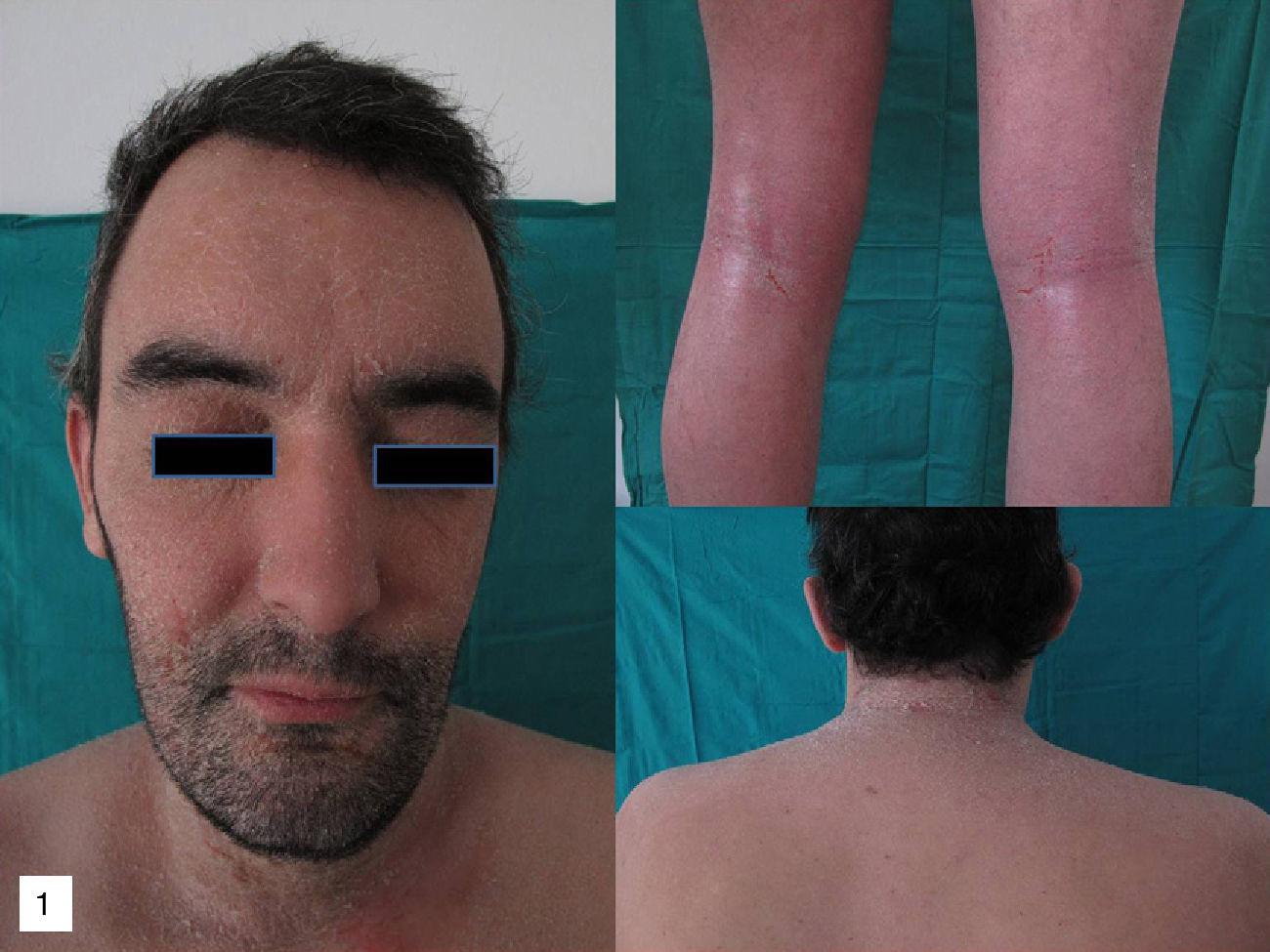
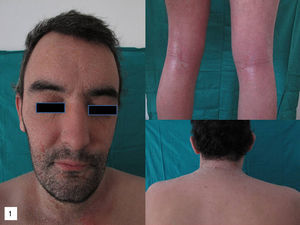
On physical examination, erythema and fine desquamation were observed on the face, trunk, and in the limb flexures; almost 50% of the body surface area was affected (Fig. 1). There were no alterations of vital signs and respiratory function was not affected.
Additional tests did not reveal eosinophilia or elevation of immunoglobulin (Ig) E or acute phase reactants (C-reactive protein, erythrocyte sedimentation rate). Moderate spongiosis and a predominantly lymphocytic perivascular infiltrate were observed on histology. The absence of necrotic keratinocytes, the minimal damage to the basal layer, and the deep lymphocytic infiltrate with no eosinophils, together with the clinical manifestations, were more suggestive of a relapse of his AD than of a toxic dermatitis.
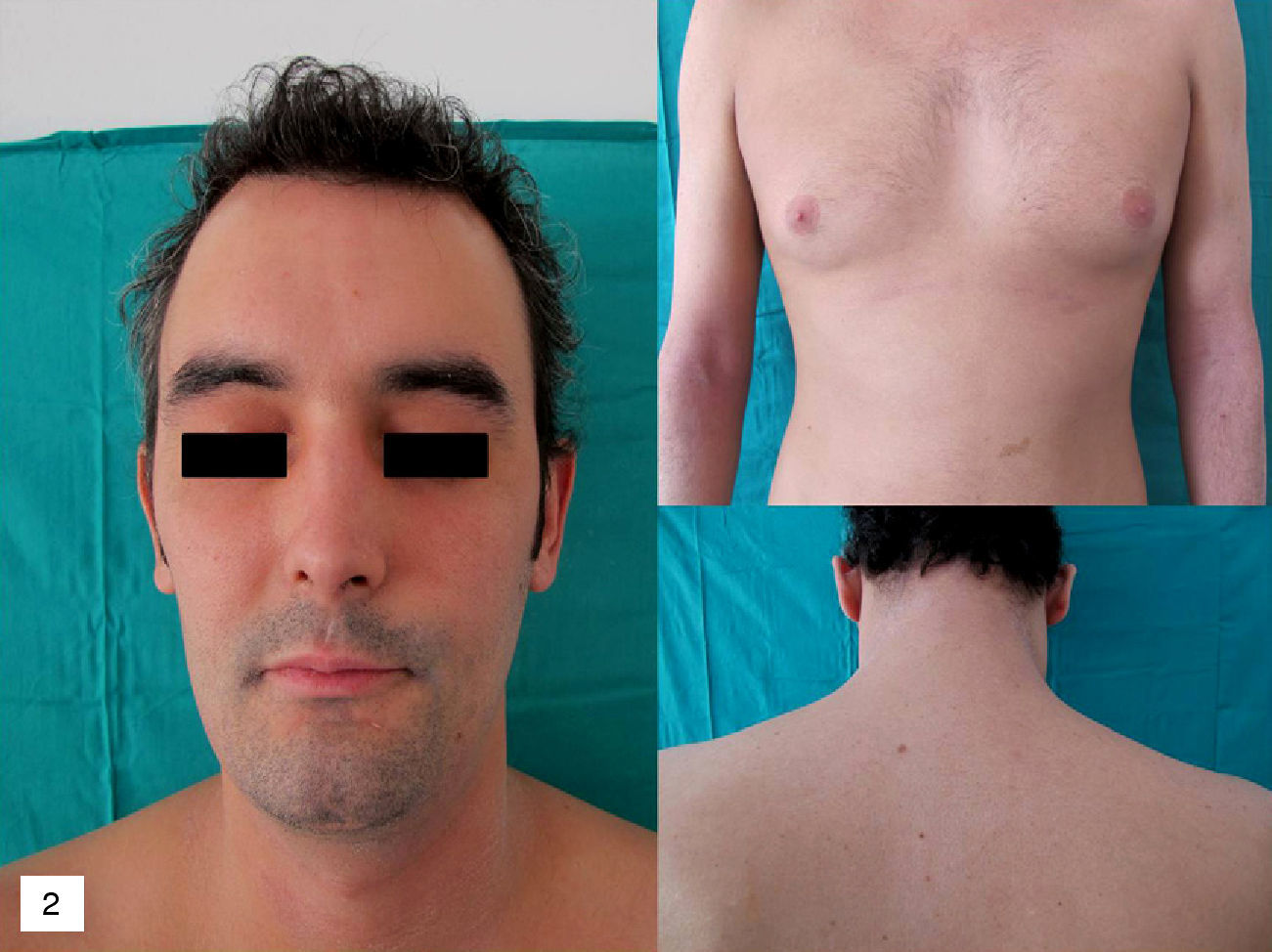
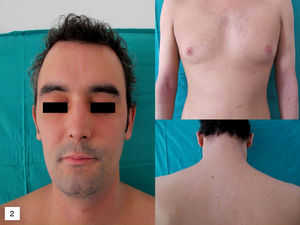
As a precaution it was decided to interrupt treatment with infliximab and start prednisone 0.5mg/kg/d in a slowly tapering regimen. Control of the skin condition was achieved in 3 weeks (Fig. 2).
The etiology and pathogenesis of AD is characterized by an acute phase with an inflammatory pattern involving type 2 helper T (TH2) cells (often with elevated IgE levels and eosinophil counts), and a chronic phase with a TH1 inflammatory pattern. It is therefore to be expected that the anti-tumor necrosis factor (TNF) agents used in psoriasis would improve the chronic forms of AD but that there would be no clear response in the acute phase.3
In the chronic phase of AD there is elevation of serum TNF-α, which is released initially by mast cells and subsequently by the TH lymphocytes and keratinocytes. The release of the cytokine further stimulates the inflammatory cascade, leading to higher levels of interleukins 1, 6, and 8, intercellular adhesion molecule 1, and vascular cell adhesion molecule. The clinical and pathological progression of a TH2 inflammatory pattern to a TH1 pattern could explain the successful response to therapy in some series of atopic patients.2 However, it would appear likely that additional, unidentified etiological and pathogenic factors are involved as, in the majority of reported cases, the response is limited to the induction phase.3
In our review of the literature we were interested to find 7 well-documented cases of relapses of AD or AD-like disease precipitated by infliximab.4–7 It is possible that more have not been reported because some relapses of AD may have been categorized as nonspecific eczema (AD has been considered to be a predictive factor for the appearance of eczema-like conditions in patients with psoriasis who receive infliximab),8 because it is an underdiagnosed adverse effect, or because few cases have required aggressive treatment.8,9 The details of the cases we found and the case we report are summarized in Table 1.
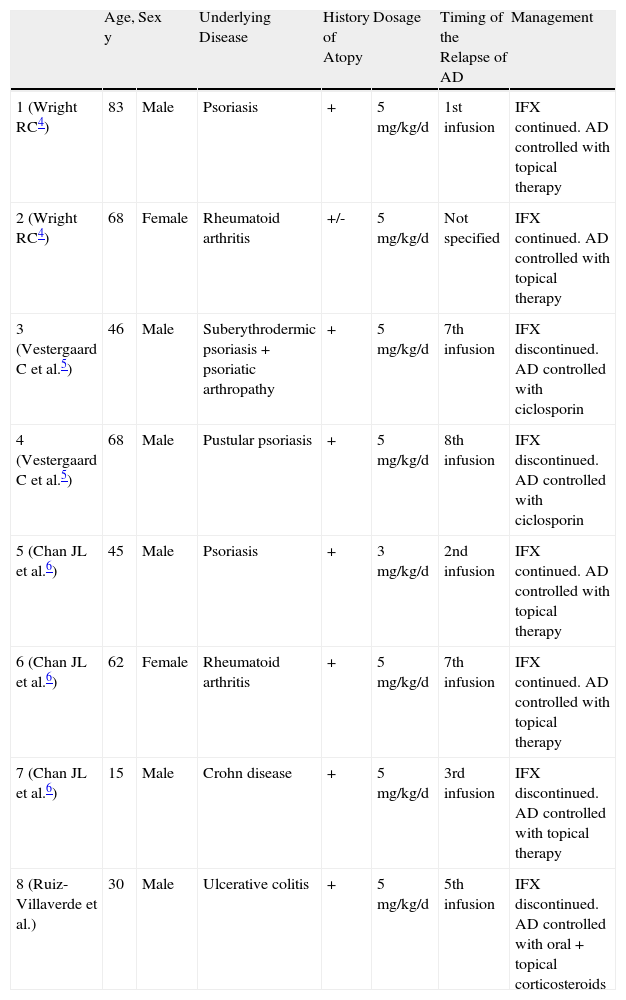
Patient and Clinical Characteristics and Course of Relapses of Atopic Dermatitis Precipitated by Infliximab.
| Age, y | Sex | Underlying Disease | History of Atopy | Dosage | Timing of the Relapse of AD | Management | |
| 1 (Wright RC4) | 83 | Male | Psoriasis | + | 5mg/kg/d | 1st infusion | IFX continued. AD controlled with topical therapy |
| 2 (Wright RC4) | 68 | Female | Rheumatoid arthritis | +/- | 5mg/kg/d | Not specified | IFX continued. AD controlled with topical therapy |
| 3 (Vestergaard C et al.5) | 46 | Male | Suberythrodermic psoriasis+psoriatic arthropathy | + | 5mg/kg/d | 7th infusion | IFX discontinued. AD controlled with ciclosporin |
| 4 (Vestergaard C et al.5) | 68 | Male | Pustular psoriasis | + | 5mg/kg/d | 8th infusion | IFX discontinued. AD controlled with ciclosporin |
| 5 (Chan JL et al.6) | 45 | Male | Psoriasis | + | 3mg/kg/d | 2nd infusion | IFX continued. AD controlled with topical therapy |
| 6 (Chan JL et al.6) | 62 | Female | Rheumatoid arthritis | + | 5mg/kg/d | 7th infusion | IFX continued. AD controlled with topical therapy |
| 7 (Chan JL et al.6) | 15 | Male | Crohn disease | + | 5mg/kg/d | 3rd infusion | IFX discontinued. AD controlled with topical therapy |
| 8 (Ruiz-Villaverde et al.) | 30 | Male | Ulcerative colitis | + | 5mg/kg/d | 5th infusion | IFX discontinued. AD controlled with oral+topical corticosteroids |
Abbreviations: AD: atopic dermatitis; IFX, infliximab.
Almost all patients in the previously published cases had a history of AD and they only differed in the underlying disease for which infliximab had been prescribed. There is no clear pattern that defines the moment at which these reactions may develop, although all occurred within the first 2 years of treatment in these cases. Our patient required systemic treatment and we believe it is important to highlight that, despite the extent of his AD, the changes in laboratory findings in previous reports did not develop in our patient.
Although our understanding of biologic therapies and the etiologic and pathogenic factors of AD increases daily, we are still unable to predict the onset of this type of adverse effect, which is surprising from a pathophysiological point of view. We hope that our presentation of this case will draw attention to the need to take a meticulous dermatology history in patients with AD who are candidates for biologic therapy, particularly in patients with rheumatic diseases or inflammatory bowel disease.
Ethical ResponsibilitiesProtection of persons and animals: The authors declare that the procedures followed adhere to the ethical guidelines of the responsible committee on human experimentation and comply with the Declaration of Helsinki of the World Medical Association. Data confidentiality: The authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study. Right to privacy and informed consent: The authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
Please cite this article as: Ruiz-Villaverde R, Galán-Gutierrez M. Exacerbación de dermatitis atópica en paciente tratado con infliximab. Actas Dermosifiliogr.2012;103:743-746.