In the past decade, the advent of biologic drugs has led to major change in the treatment of patients with moderate-to-severe psoriasis.
Traditionally, transplant patients have been excluded from clinical trials for safety reasons (risk of infection, risk of secondary cancer, and risk of induced autoimmunity) and there is therefore very little evidence regarding the use of these drugs in transplant patients; the information published to date consists of isolate clinical cases or case series.1 As a result, experience of their use in this group of patients is scarce.
We report the clinical case of a kidney-transplant patient with severe psoriasis and good response to treatment with etanercept.
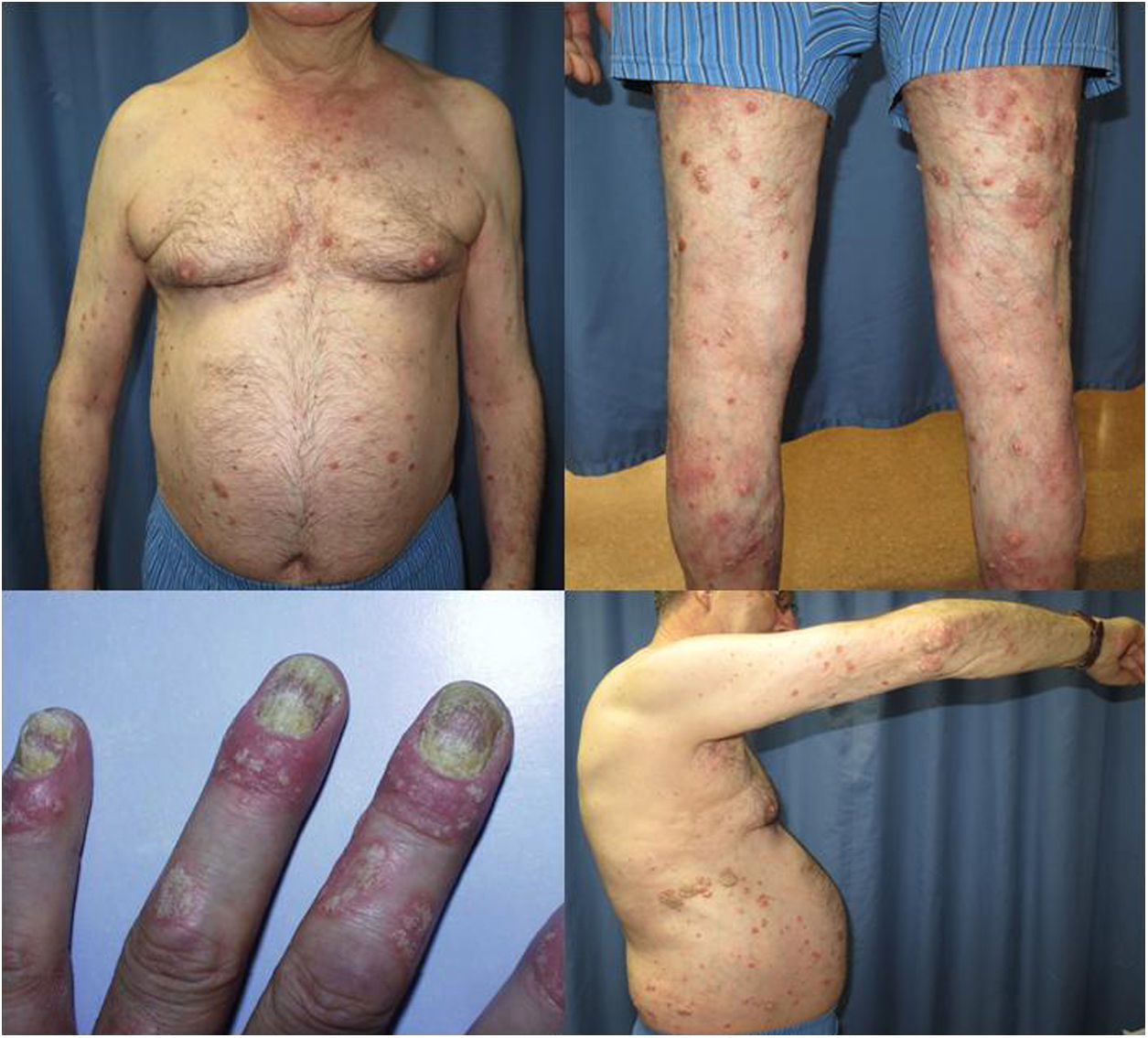
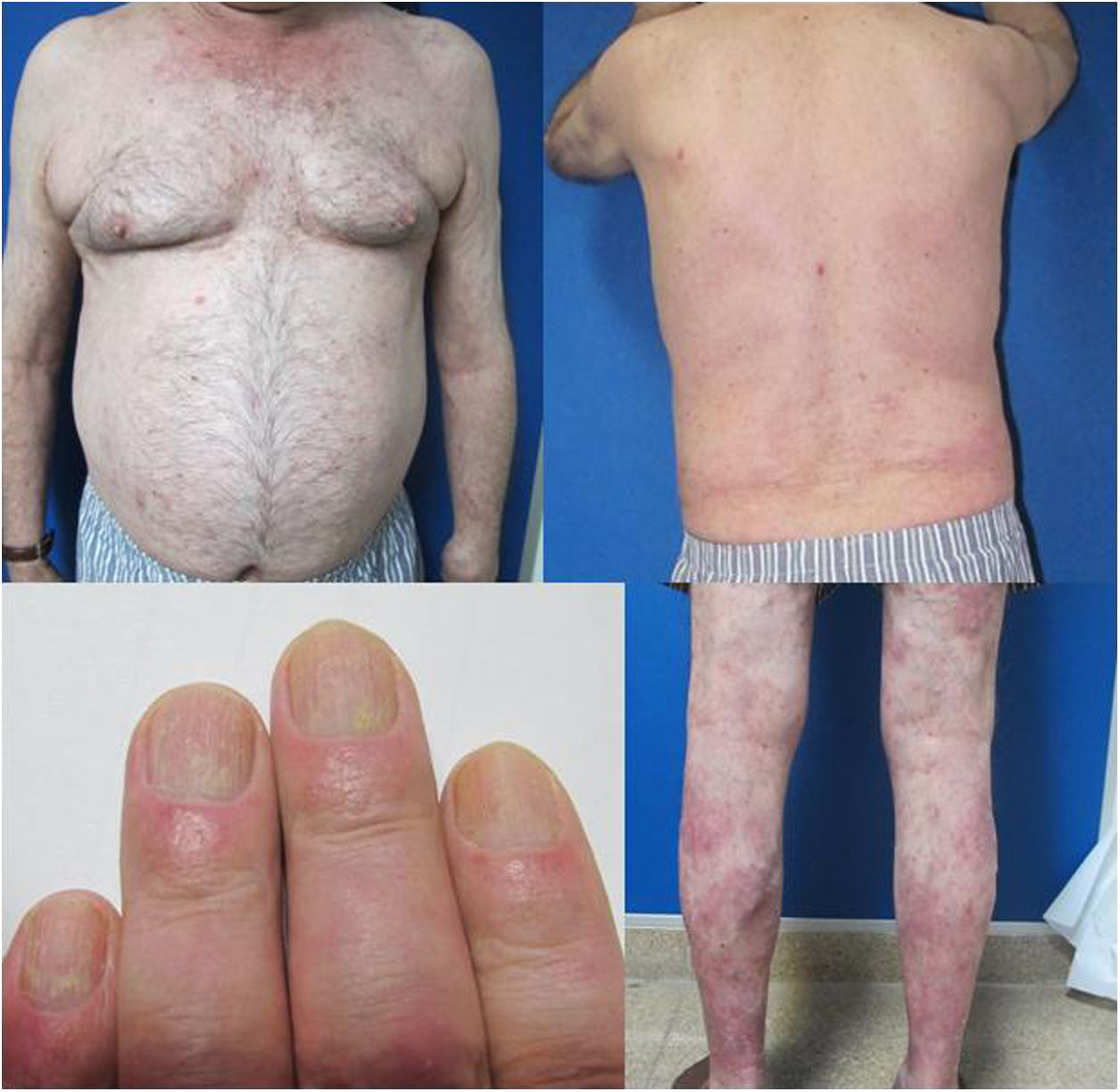
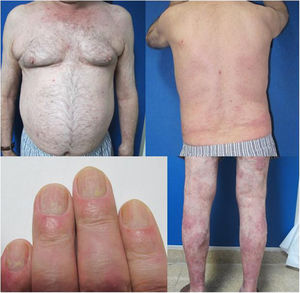
The patient was a 67-year-old man with severe psoriasis, who had received different treatments since adolescence: topical corticosteroids, acitretin, narrow-band ultraviolet (NB-UVB) phototherapy, cyclosporin A, and oral methotrexate. The patient’s personal history included hypertension and hyperlipidemia, both adequately controlled with treatment. The patient had undergone a kidney transplant in August 2003, due to chronic interstitial nephritis. Since then, he had been undergoing treatment with variable dosages of corticosteroids (10−20 mg/d) and topical corticosteroids, together with his immunosuppressant regimen (tacrolimus, 3 mg/12 h and mofetil mycophenolate, 1 g/24 h). In 2012, the patient underwent a transurethral resection of a high-grade (pT1G2) gallbladder tumor with subsequent intravesical instillation of mitomycin C; the tumor is currently in complete remission. Because of the poor control of his psoriasis lesions, with a Psoriasis Area and Severity Index (PASI) score of 15 and a Body Surface Area (BSA) of 8%, with impaired quality of life, treatment with etanercept at a dosage of 50 mg/wk was instated in August 2017; the mycophenolate was stopped and the oral tacrolimus was continued (Fig. 1). A significant improvement in the skin lesions was achieved 12 weeks after starting treatment with etanercept, with a reduction of the PASI score to 2 and the BSA to 1% (Fig. 2). Currently, after a year of follow-up, the patient continues in treatment with etanercept at a dosage of 50 mg/wk, with adequate control of the disease (PASI, 0; BSA, 0%) and no evidence of adverse effects or signs of rejection (estimated CKD glomerular filtration rate >60 mL/min/1.73 m2; urea, 38 mg/dL; and creatinine, 1.15 mg/dL [0.70–1.30]).
To date, very few cases have been reported in the literature of transplant patients in treatment with biologic drugs. In most of them, biologic drugs have been used in these patients to control inflammatory bowel disease (Crohn disease or ulcerative colitis) or rheumatologic diseases (rheumatoid arthritis or ankylosing spondylitis).1
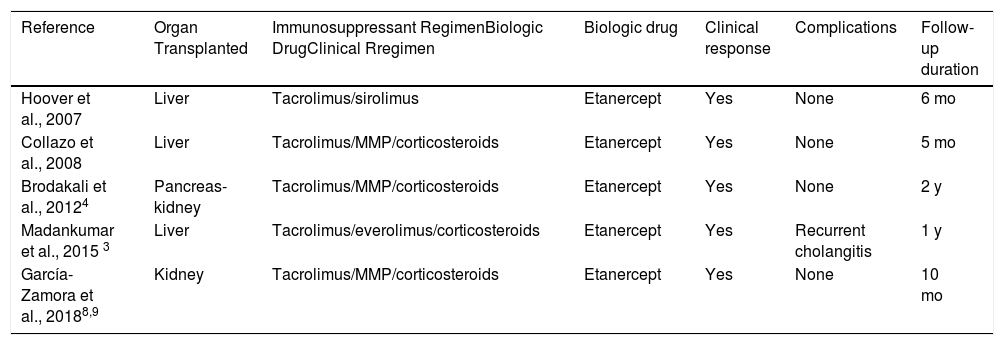
We found only 4 cases in the literature of patients with a solid-organ transplant in treatment with biologic drugs to control psoriasis. These cases are shown in Table 1. In all cases, the drug used was etanercept, probably due to the extensive experience with its use and its good safety profile, which allowed for good clinical control, good tolerance, and no evidence of rejection; however, follow-up was limited in all cases (5–24 months).2–4 In 1 case, the treatment with the drug had to be interrupted on several occasions due to recurrent episodes of cholangitis.3
With Solid-Organ Transplant and Psoriasis Treated with Anti-TNFα to Control the Psoriasis.
| Reference | Organ Transplanted | Immunosuppressant RegimenBiologic DrugClinical Rregimen | Biologic drug | Clinical response | Complications | Follow-up duration |
|---|---|---|---|---|---|---|
| Hoover et al., 2007 | Liver | Tacrolimus/sirolimus | Etanercept | Yes | None | 6 mo |
| Collazo et al., 2008 | Liver | Tacrolimus/MMP/corticosteroids | Etanercept | Yes | None | 5 mo |
| Brodakali et al., 20124 | Pancreas-kidney | Tacrolimus/MMP/corticosteroids | Etanercept | Yes | None | 2 y |
| Madankumar et al., 2015 3 | Liver | Tacrolimus/everolimus/corticosteroids | Etanercept | Yes | Recurrent cholangitis | 1 y |
| García-Zamora et al., 20188,9 | Kidney | Tacrolimus/MMP/corticosteroids | Etanercept | Yes | None | 10 mo |
Abbreviation: MMP indicates mofetil mycophenolate.
In the largest series published to date of kidney-transplant patients in treatment with anti-TNFα drugs, the authors report a high rate of severe infections (50%); this has not been reported in other case series.1 Garrouste et al.1 report a particularly high risk of infection in patients undergoing immunosuppressant treatment combined with corticosteroids at doses above 10 mg/d for the first 3 months after starting the anti-TNFα drug.
We found no reports in the literature of patients with a solid-organ transplant in treatment with non-anti–TNFα biologic drugs to control psoriasis. Only 1 case has been reported, of a 44-year-old man with Crohn disease and recipient of a liver transplant due to cryptogenic cirrhosis, who was treated with ustekinumab to control the inflammatory bowel disease, with a good response and no adverse effects after 12 months of follow-up.5
The literature contains little evidence of the need to modify the immunosuppressant regimen in these patients when the biologic drug is introduced. Many of the reported cases do not mention any modification of the immunosuppressant treatment. In our case, we decided to suspend the mofetil mycophenolate and maintain the tacrolimus at 3 mg/12 h, together with the etanercept. Furthermore, anti-TNF drugs are being used off-label as immunosuppressants in recipients of solid-organ transplants.6,7
In conclusion, with this new case, we provide additional information on the use of biologic drugs in transplant patients. Biologic drugs may be used in this type of patient provided that the patients are carefully selected and closely observed for potential infections, although we believe that more cases and studies are needed to corroborate this.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: García-Zamora E., Gómez de la Fuente E., Miñano-Medrano R., López-Estebaranz JL. Uso de etanercept en un paciente trasplantado renal con psoriasis. Actas Dermosifiliogr. 2020;111:169–171.