Electrochemotherapy is indicated for the treatment of unresectable cutaneous and subcutaneous tumors. The technique involves the synergistic use of electroporation of cell membranes to increase the cytotoxicity of anticancer drugs delivered to the tumor cells. The aim of this study was to analyze the clinical effectiveness and safety of electrochemotherapy in the treatment of unresectable locoregional recurrent or metastatic melanomas.
Material and methodsWe studied 31 patients treated between January 2007 and December 2012. The European Standard Operating Procedures of Electrochemotherapy (ESOPE) were applied in all cases. Treatment response was analyzed as overall patient response (mean response based on results for all lesions treated in a given patient).
ResultsResponse was classified as partial in 49% of patients and complete in 23%. At 1 year, the level of response achieved had been maintained in 17 patients. Disease progression was observed in 28% of the series. Immediate local complications (pain, swelling, erythema) were mild and resolved within 48hours in most cases. Eight patients developed subsequent local complications, such as ulcers and secondary infections associated with necrosis of the lesions. These complications were brought under control with topical treatments.
ConclusionsElectrochemotherapy is a very effective, safe, and efficient treatment for advanced locoregional disease in patients with unresectable melanoma lesions.
La electroquimioterapia (EQT) es una técnica terapéutica indicada en tumores cutáneos y subcutáneos no resecables quirúrgicamente. La EQT se fundamenta en la acción sinérgica de un fármaco antineoplásico junto con la electroporación de las membranas celulares para aumentar su citotoxicidad. El objetivo del presente estudio es objetivar la eficacia clínica, así como el perfil de seguridad de la EQT como tratamiento en pacientes con recidivas o metástasis cutáneas locorregionales de melanoma no abordables quirúrgicamente.
Material y métodosEntre enero de 2007 y diciembre de 2012 se incluyeron 31 pacientes. Todos los tratamientos se realizaron siguiendo las guías de consenso European Standard Operating Procedures of Electrochemotherapy (ESOPE). La respuesta se calculó por paciente, obteniendo la media del conjunto de las lesiones.
ResultadosEn el 49% de los casos se demostró una respuesta parcial y en el 23% se obtuvo una respuesta completa. Diecisiete pacientes mantuvieron la respuesta al año de seguimiento. En el 28% existió progresión de la enfermedad. Las complicaciones locales inmediatas (dolor, edema, eritema) fueron leves, y se resolvieron en las primeras 48h en la mayoría de los casos. Ocho pacientes presentaron complicaciones locales posteriores, como ulceración y sobreinfección, secundarias a la necrosis de las lesiones y fueron controladas con tratamientos tópicos.
ConclusionesLa EQT presenta un excelente perfil de eficacia, eficiencia y seguridad, siendo de gran utilidad en el control de la enfermedad locorregional avanzada en el melanoma en lesiones no resecables quirúrgicamente.
Electrochemotherapy (ECT) is a novel therapeutic option indicated for the treatment of unresectable cutaneous and subcutaneous tumors when, due to the site or extent of the disease, the initial treatment fails or no other treatment options are available.1 This technique combines the electroporation of cell membranes with the administration of anticancer drugs for the treatment and locoregional control of solid tumors. Since its introduction in the early 1980s, electroporation has been used both in vitro and in vivo to deliver water-soluble substances such as nucleotides, peptides, and drugs to the cell interior.2 Electroporation is a physical-chemical technique in which the cell membrane is subjected to a pulsed, high-intensity electrical field, which creates transmembrane channels, or pores, through which water-soluble molecules that under normal conditions are non-permeant can be delivered into the cell cytosol.3 This temporary, reversible permeabilization of the cell membrane makes it possible to achieve a higher intracellular drug concentration. In the case of cisplatin and bleomycin, electroporation increases cytotoxicity by a factor of 100 and 1000, respectively.4
In 2006, the European Standard Operating Procedures of Electrochemotherapy (ESOPE)5 project studied the efficacy, toxicity, dose, and mechanism of action of ECT and established standard procedures for the technique. The ESOPE study was conducted at 4 European cancer centers with patients who had cutaneous metastases of various etiologies. The local tumor control rate for ECT was 88% with intravenous bleomycin, 73% with intratumoral bleomycin, and 75% with intratumoral cisplatin.
The aims of this study were to analyze the response at 1 month and 1 year as well as the possible complications related to ECT as locoregional therapy in patients with unresectable recurrences or cutaneous metastases of melanoma.
Material and MethodsThis was an observational study of a case series in which data were collected both prospectively and retrospectively between January 2007 and December 2012 at the Department of Dermatology of Hospital Clínic (Universitat de Barcelona). Patients were included in the study if they had unresectable recurrences or cutaneous metastases of melanoma or if no alternative treatment was available after the failure of previous therapies. Given the critical situation of these patients, no strict exclusion criteria were applied, with the exception of allergy to bleomycin or its derivatives. Patients who had previously received local or systemic therapies were not excluded. All treatments were carried out in accordance with the ESOPE consensus guidelines.5
The Cliniporator electroporation device (IGEA, Italy) was used. The electrodes used to treat cutaneous and subcutaneous lesions consisted of 6 needles of variable length (between 1 and 3cm). Five of the electrode needles were arranged in the shape of a pentagon, with the sixth needle occupying its geometric center. The intensity and amplitude of the electric pulses were kept higher than 400V/cm2 and 1A, respectively. Pulse length was 100ms. Amplitude, frequency, and length of pulses were managed automatically using the console of the device, which controlled the power unit in accordance with the ESOPE guidelines. In each application, 8 electric pulses with the aforementioned characteristics and a frequency of 5000Hz were produced.
In accordance with the ESOPE protocol,6 the electric pulses were started 8minutes after intravenous injection of bleomycin. The dose of bleomycin administered was 15 000IU/m2. ECT was applied under general anesthesia. Informed consent was obtained from all patients.
The lesions were photographed before and after each course of treatment. The following data were collected for each patient: site of lesions, stage of disease, histologic characteristics of primary tumor, presence or absence of distant disease (nodal disease or internal organ metastasis), previous treatments, number of courses of ECT, interval between courses of ECT, dose of bleomycin, number of pulses per session, and side effects. When necessary, patients received a new course of treatment after 4 to 6 weeks. Treatment response was assessed 4 weeks after the last cycle and 1 year after treatment using the following definitions: a) clinical progression (tumor size increase of >25%); b) locoregional disease stabilization or partial response (tumor size increase of<25% or decrease of<50%); or c) complete response (reduction >50%). Instead of determining the response of each lesion (as has been done in other studies), we determined the mean response of all lesions in each patient in order to obtain an overall result for each patient. (In patients with numerous lesions, it can be confusing to report results for each lesion.)
New lesions that subsequently appeared in untreated regions were considered to be new disease rather than recurrences of treated lesions; consequently, they were not considered in the determination of local response.
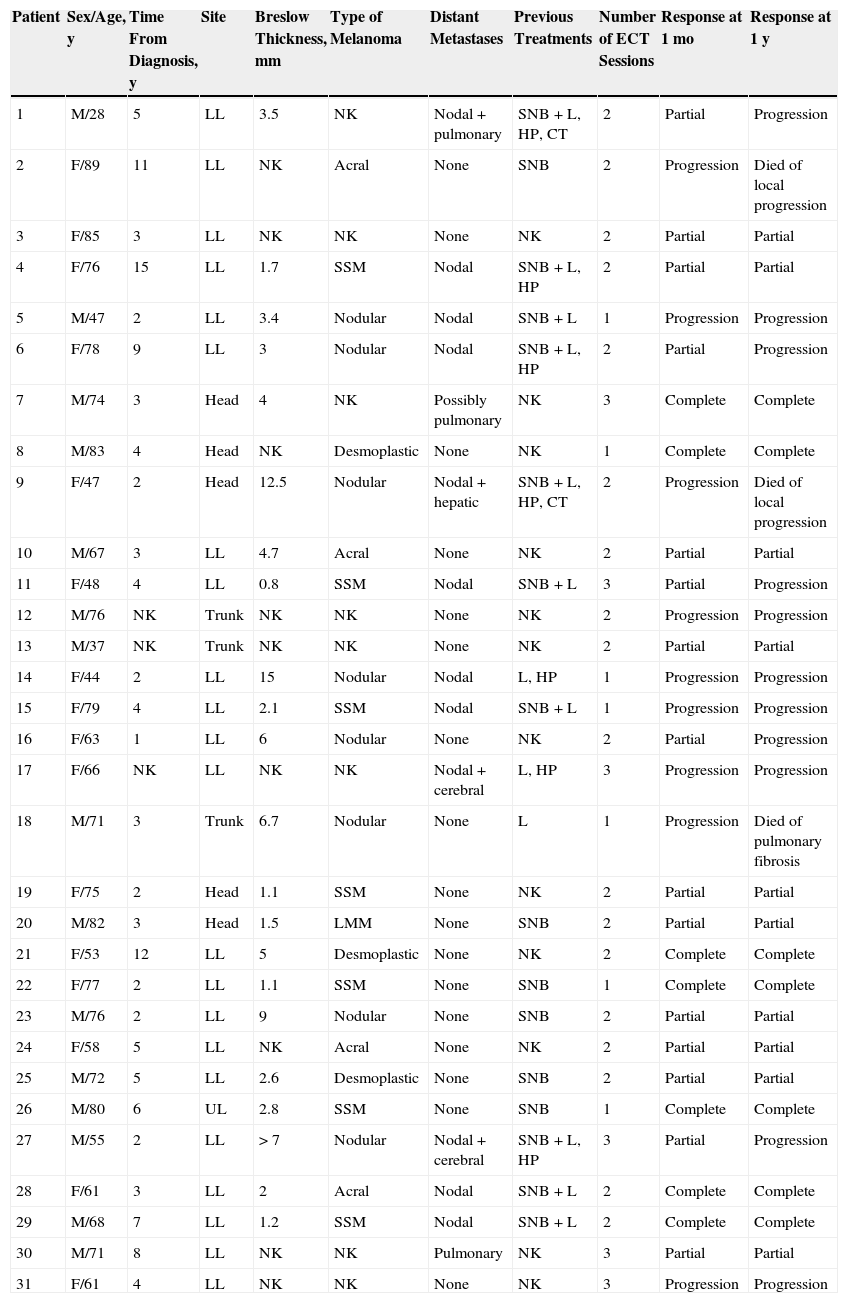
ResultsThirty-one patients (16 women and 15 men) were included in the study (Table 1). No patients were excluded. The mean age was 66 years (range, 28-89 years). The mean Breslow thickness of the primary tumor was 4.39mm (range, 0.8-15mm). The primary melanomas were of the following types: nodular (8 cases), superficial spreading (7 cases), acral (4 cases), desmoplastic (3 cases), and lentigo maligna (1 case). The type of primary melanoma could not be confirmed in 8 patients referred by other hospitals. The primary tumor was located on the lower limbs in 68% of cases. Other sites included the head and neck (19%), the trunk (10%), and the upper limbs (3%).
Baseline and Response Characteristics of 31 Patients With Melanoma Skin Metastases Treated With Electrochemotherapy.
| Patient | Sex/Age, y | Time From Diagnosis, y | Site | Breslow Thickness, mm | Type of Melanoma | Distant Metastases | Previous Treatments | Number of ECT Sessions | Response at 1 mo | Response at 1 y |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/28 | 5 | LL | 3.5 | NK | Nodal+pulmonary | SNB+L, HP, CT | 2 | Partial | Progression |
| 2 | F/89 | 11 | LL | NK | Acral | None | SNB | 2 | Progression | Died of local progression |
| 3 | F/85 | 3 | LL | NK | NK | None | NK | 2 | Partial | Partial |
| 4 | F/76 | 15 | LL | 1.7 | SSM | Nodal | SNB+L, HP | 2 | Partial | Partial |
| 5 | M/47 | 2 | LL | 3.4 | Nodular | Nodal | SNB+L | 1 | Progression | Progression |
| 6 | F/78 | 9 | LL | 3 | Nodular | Nodal | SNB+L, HP | 2 | Partial | Progression |
| 7 | M/74 | 3 | Head | 4 | NK | Possibly pulmonary | NK | 3 | Complete | Complete |
| 8 | M/83 | 4 | Head | NK | Desmoplastic | None | NK | 1 | Complete | Complete |
| 9 | F/47 | 2 | Head | 12.5 | Nodular | Nodal+hepatic | SNB+L, HP, CT | 2 | Progression | Died of local progression |
| 10 | M/67 | 3 | LL | 4.7 | Acral | None | NK | 2 | Partial | Partial |
| 11 | F/48 | 4 | LL | 0.8 | SSM | Nodal | SNB+L | 3 | Partial | Progression |
| 12 | M/76 | NK | Trunk | NK | NK | None | NK | 2 | Progression | Progression |
| 13 | M/37 | NK | Trunk | NK | NK | None | NK | 2 | Partial | Partial |
| 14 | F/44 | 2 | LL | 15 | Nodular | Nodal | L, HP | 1 | Progression | Progression |
| 15 | F/79 | 4 | LL | 2.1 | SSM | Nodal | SNB+L | 1 | Progression | Progression |
| 16 | F/63 | 1 | LL | 6 | Nodular | None | NK | 2 | Partial | Progression |
| 17 | F/66 | NK | LL | NK | NK | Nodal+cerebral | L, HP | 3 | Progression | Progression |
| 18 | M/71 | 3 | Trunk | 6.7 | Nodular | None | L | 1 | Progression | Died of pulmonary fibrosis |
| 19 | F/75 | 2 | Head | 1.1 | SSM | None | NK | 2 | Partial | Partial |
| 20 | M/82 | 3 | Head | 1.5 | LMM | None | SNB | 2 | Partial | Partial |
| 21 | F/53 | 12 | LL | 5 | Desmoplastic | None | NK | 2 | Complete | Complete |
| 22 | F/77 | 2 | LL | 1.1 | SSM | None | SNB | 1 | Complete | Complete |
| 23 | M/76 | 2 | LL | 9 | Nodular | None | SNB | 2 | Partial | Partial |
| 24 | F/58 | 5 | LL | NK | Acral | None | NK | 2 | Partial | Partial |
| 25 | M/72 | 5 | LL | 2.6 | Desmoplastic | None | SNB | 2 | Partial | Partial |
| 26 | M/80 | 6 | UL | 2.8 | SSM | None | SNB | 1 | Complete | Complete |
| 27 | M/55 | 2 | LL | >7 | Nodular | Nodal+cerebral | SNB+L, HP | 3 | Partial | Progression |
| 28 | F/61 | 3 | LL | 2 | Acral | Nodal | SNB+L | 2 | Complete | Complete |
| 29 | M/68 | 7 | LL | 1.2 | SSM | Nodal | SNB+L | 2 | Complete | Complete |
| 30 | M/71 | 8 | LL | NK | NK | Pulmonary | NK | 3 | Partial | Partial |
| 31 | F/61 | 4 | LL | NK | NK | None | NK | 3 | Progression | Progression |
Abbreviations: CT, chemotherapy; ECT, electrochemotherapy; F, female; HP, hyperthermic perfusion; L, lymphadenectomy; LL, lower limbs; LMM: lentigo maligna melanoma; M, male; NK, not known; SNB, sentinel node biopsy; SSM, superficial spreading melanoma; UL, upper limbs.
A total of 57 ECT sessions were carried out. All patients received at least 1 ECT session; 18 patients had 2 sessions and only 6 had 3 sessions. The number of pulses administered per lesion was determined as a function of lesion size: 2 pulses for lesions less than 5mm in diameter and 3 to 5 pulses for larger lesions. Five patients developed solid organ metastases before or during the study. The previous treatments received by the patients were as follows: a) sentinel node biopsy, 16 cases (with subsequent lymphadenectomy in 10 cases); b) lymphadenectomy, 13 cases (without previous sentinel node biopsy in 3 cases); c) hyperthermic isolated limb perfusion, 7 cases; and d) systemic chemotherapy, 2 cases.
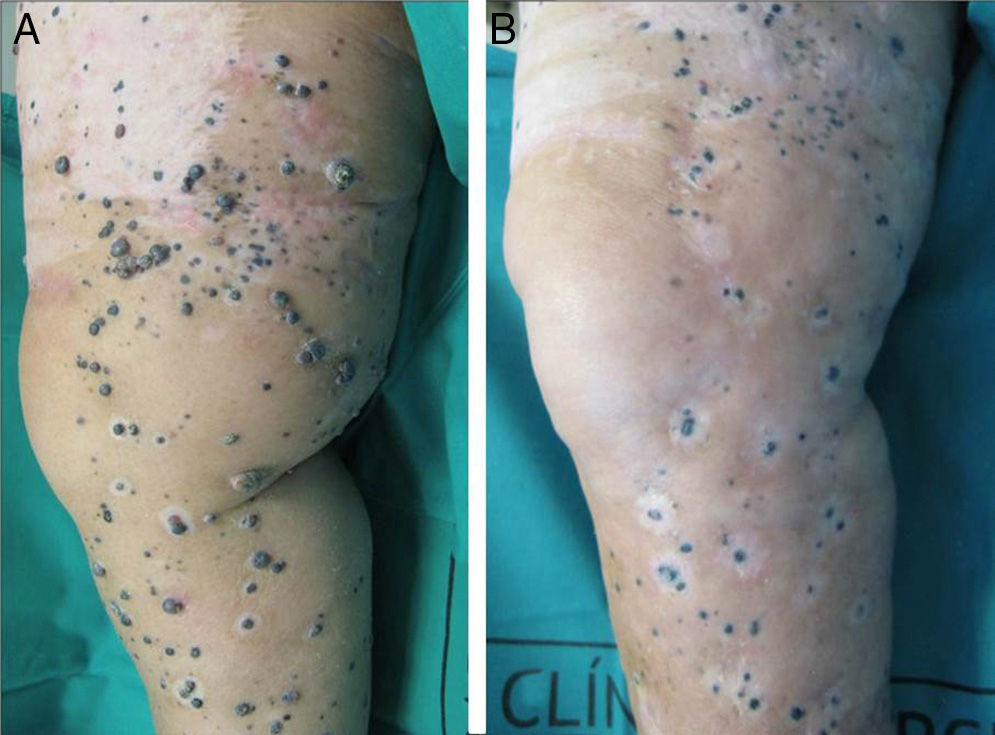
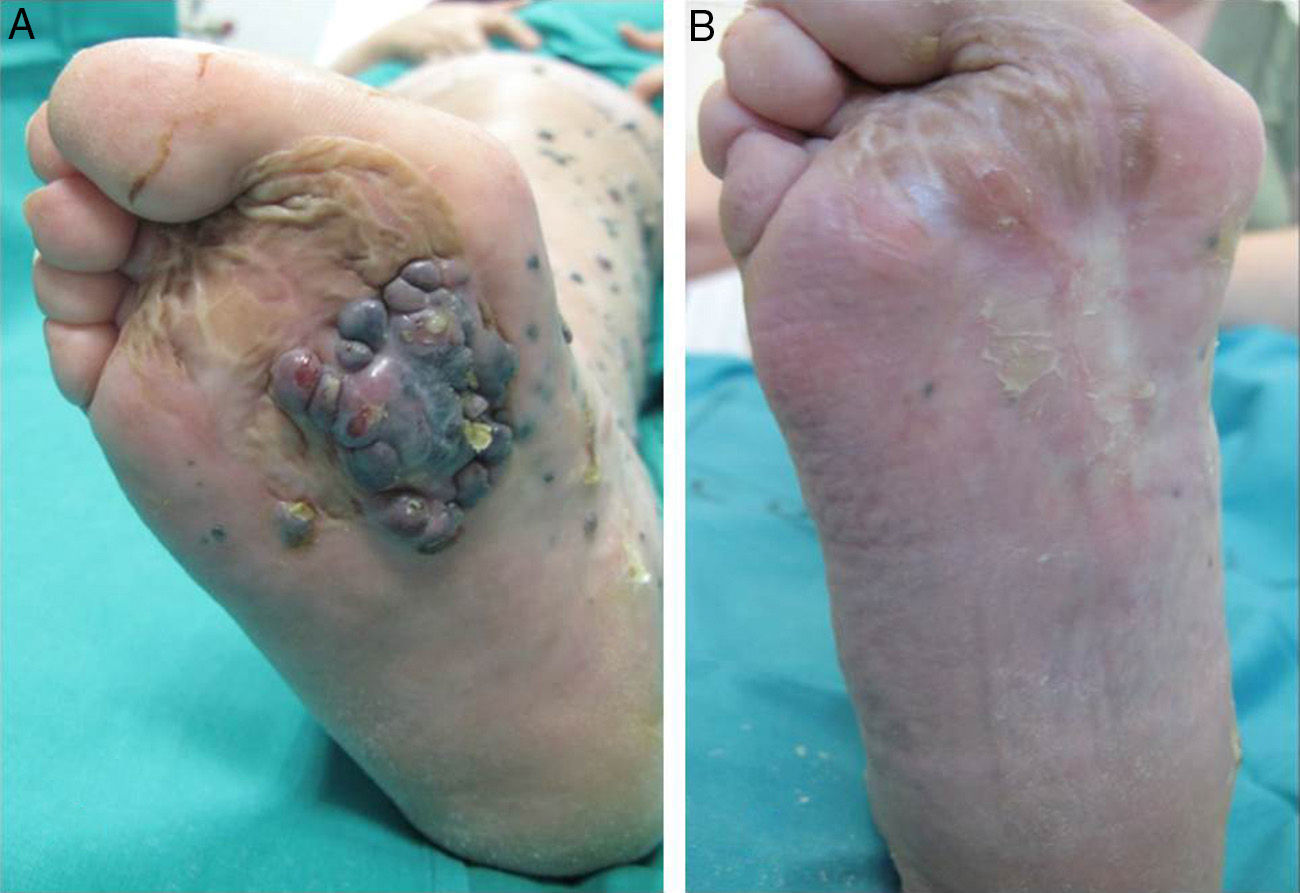
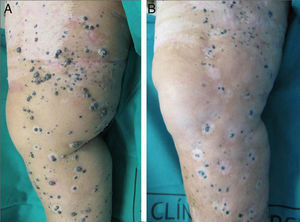
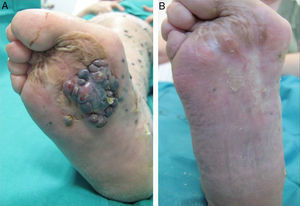
A positive response was observed in 22 patients (72%), with improvement or stabilization of the lesions for at least 12 months of follow-up (Figs. 1 and 2). Of the patients who had a positive response, clinical stabilization occurred in 15 cases (49%) and a complete clinical response of the treated lesions was seen in the remaining 7 cases (23%). Disease progression occurred after the ECT cycle in 28% of cases. In 5 of the 15 patients with a partial response, disease progression occurred 8 to 12 months after treatment.
The treatment was well tolerated by most patients and no major complications occurred. The most common systemic side effects were nausea and vomiting, and these were well controlled with medical treatment. Immediate local complications were mild, occurred in all patients, and in most cases resolved within 48hours. These complications included pain, edema, erythema, and oozing. Eight patients had subsequent local complications such as ulceration and superinfection secondary to necrosis of the lesions. These complications were controlled with topical treatments. One patient died of progressive respiratory failure in the first 7 days of follow-up; no relationship to the ECT was demonstrated.
DiscussionIn-transit melanoma metastases are metastatic deposits that occur along the skin between the site of the primary tumor and the regional lymph node group. They have been reported in 5% of patients free of nodal disease and in as many as 20% of patients with lymphatic metastases.7 The management of multiple cutaneous recurrences is a therapeutic challenge that affects up to 80% of patients who have melanoma recurrences in the first 5 years of follow-up.8 Although surgical resection is the treatment of choice, this option is not feasible in many cases due to the site or the large number of lesions. Progression of advanced locoregional disease substantially worsens quality of life in these patients because of the torpid progression of the lesions, which cause pain and functional impairment in the affected region. Additionally, the lesions ulcerate, bleed, and increase in number and size, and secondary infection can occur; palliative treatments are therefore necessary. Unfortunately, however, there is no consensus regarding the management of these cases because the available therapeutic options are limited, very expensive, and have variable results. As a result, the prognosis is poor and 5-year survival rates range from 12% to 37%.9 There are various treatment options: imiquimod,10 ECT, systemic chemotherapy,11 radiotherapy,12 intralesional interleukin-2,13 interferon alfa,14 intratumoral bacillus Calmette-Guérin,15 and hyperthermic isolated limb perfusion.16,17 These treatments have produced variable results.
ECT is a novel option for treating unresectable cutaneous and subcutaneous tumors of various etiologies when previous treatments have failed. The utility of ECT has been reported in melanoma,18,19 Kaposi sarcoma,20 basal cell carcinoma,21 and squamous cell carcinoma.1 In a recent study, 3 cases of primary cutaneous marginal zone B-cell lymphoma responded to ECT.22 Positive responses have also been reported in cutaneous and subcutaneous metastases of gastric cancer,23 breast cancer,24 and squamous cell carcinoma of the head and neck. The applicability of ECT with longer electrodes and other systems is currently being investigated in deeper-seated lesions such as bone metastases, soft-tissue sarcomas,25 and colorectal liver metastases.26
Very high overall response rates have been reported in cutaneous melanoma metastases. One study reported a partial response in 39% of cases and a complete response in 23% of cases (61% overall response rate).27 Another study reported a complete response in 58% of cases and a partial response in 35% of cases (93% overall response rate).28 In our study, partial response occurred in 49% of cases and complete response in 23% of cases (72% overall response rate). Disease progression occurred in 28% of cases. Some authors have observed better responses in nodules < 2cm.29 In melanoma, ECT is currently only used as palliative treatment because it does not alter the progression of metastases in internal organs and has not been shown to increase survival.30 However, Caracò et al31 reported that 21.7% of patients had a long-lasting complete response after a mean follow-up of 27.5 months. Locoregional use of ECT could therefore play an important role in the medium term, in addition to controlling the disease and preventing complications in the short term. In addition, because the secondary effects associated with ECT are minimal, patients can be retreated until a better response is achieved.28 In our case series, we also found that the positive results are maintained for at least 1 year in most cases.
The side effects of ECT tend to be mild and transient. In our experience, complications such as erythema, edema, and pain always appear immediately and resolve within a few days of the procedure. Nausea and vomiting require special attention because of the discomfort they cause. Myoclonias have been reported in some case series.30 Late side effects and complications have rarely been reported. It is therefore interesting that 8 of our patients had late local complications such as ulceration and secondary infections associated with necrosis of the lesions; these complications were controlled with topical treatments. Moreover, bleomycin is known to induce a wide variety of respiratory and pulmonary dysfunctions, including pulmonary fibrosis.32 Systemic toxicity is low in ECT because the bleomycin doses administered systemically are relatively low (the very high intratumoral concentrations of cytostatic agents are a result of electroporation). Nevertheless, 1 patient in our study died from a worsening of underlying pulmonary fibrosis. We therefore suggest the use of cisplatin in patients with underlying pulmonary alterations.
Due to its high response rate, lower morbidity, and lower cost, ECT is starting to replace hyperthermic isolated limb perfusion as the first choice in the treatment of locoregional melanoma metastases in the limbs.33 ECT also has a hemostatic effect. It reduces blood flow, which, in addition to having an antitumor effect, makes it possible to immediately control bleeding in advanced skin tumors,34 a frequent complication that worsens quality of life considerably. Some researchers have successfully used ECT to stop bleeding in refractory melanoma metastases.35
In conclusion, ECT is a palliative yet highly useful option for controlling recurrent melanoma or multiple unresectable cutaneous melanoma metastases when the initial treatment fails or when no other treatment options exist due to the site or extent of the disease. In the near future, however, ECT may become the therapy of choice in advanced locoregional disease due to its excellent profile of efficacy, efficiency, and safety.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: Mir-Bonafé J, Vilalta A, Alarcón I, Carrera C, Puig S, Malvehy J, et al. Electroquimioterapia en metástasis cutáneas de melanoma: Experiencia en 31 casos. Actas Dermosifiliogr. 2015;106:285–291.