Atopic dermatitis has become a health problem in our setting due to its rising prevalence, impact on quality of life, associated costs, and role in the progression to other atopic diseases. Furthermore, atopic dermatitis has no definitive cure and therefore preventive measures are important. In this article, we review the latest advances in both primary prevention (reduction of the incidence of atopic dermatitis) and secondary prevention (reduction of associated morbidity and reduction of the atopic march). We analyze the different preventive strategies available, including modification of the immune system through microbial exposure, induction of immune tolerance through antigen exposure, and restoration of skin barrier function to halt the atopic march. Dermatologists need to be familiar with these strategies in order to apply them where necessary and to accurately inform patients and their relatives to prevent misguided or inappropriate actions.
La dermatitis atópica (DA) se ha convertido en un problema de salud en nuestro medio debido al aumento de su prevalencia, la alteración de la calidad de vida, los gastos que ocasiona y su implicación en la progresión a otras enfermedades atópicas. Además no tiene una cura definitiva, por lo que sería interesante la aplicación de medidas preventivas. En este artículo se revisan los últimos avances implicados en su prevención, tanto primaria (disminución de incidencia), como secundaria (disminución de morbilidad, evitar la progresión de la marcha atópica). Se abordan las diversas estrategias implicadas, entre las que se encuentran la modificación del sistema inmune mediante exposición microbiana, la inducción de tolerancia inmunológica por exposición antigénica y la restauración de la función barrera para frenar la marcha atópica. Estas medidas deberían ser conocidas por los dermatólogos para aplicarlas cuando sea posible, y poder informar adecuadamente a los pacientes y familiares, evitando actuaciones inadecuadas y/o erróneas.
Atopic dermatitis (AD) is a chronic inflammatory disease whose prevalence has increased considerably in recent years, with the consequent marked effect on patient quality of life and very high costs. Furthermore, it is usually the first manifestation of the so-called atopic march, and a high percentage of patients with AD eventually develop food allergy, asthma, and/or allergic rhinitis. Approximately, one-third of patients with AD will develop asthma and two-thirds allergic rhinitis; however, there is no conclusive evidence on the percentage who will develop food allergy.1,2 Therefore, AD has become a real health problem in our setting. Since AD is a chronic disease with no cure, it would be extremely interesting to have effective preventive measures. Based on a critical review of available scientific evidence and levels of evidence for specific interventions (Table 1),3 the present article aims to determine whether there are effective ways of preventing AD.
Levels of Evidence.
| Level of Evidence | |
|---|---|
| 1a | Meta-analysis of well-designed randomized controlled clinical trials |
| 1b | Individual randomized controlled trial |
| 2a | Systematic review of cohort studies |
| 2b | Individual cohort study. Individual low-quality randomized controlled trial |
| 3a | Systematic review of case-control studies |
| 3b | Individual case-control study |
| 4 | Case series, poor quality cohort case-control studies |
| 5 | Expert opinions or documents |
Source: Sackett and Wennberg.3
Prevention can be at several levels. Here, interventions will be reviewed at primary and secondary level. By primary prevention, we mean the set of actions aimed at preventing onset of the disease; by secondary prevention, we mean early diagnosis and treatment to reduce morbidity and/or mortality. In the former, we try to reduce the incidence of DA, and in the latter, we try to reduce severity and complications.
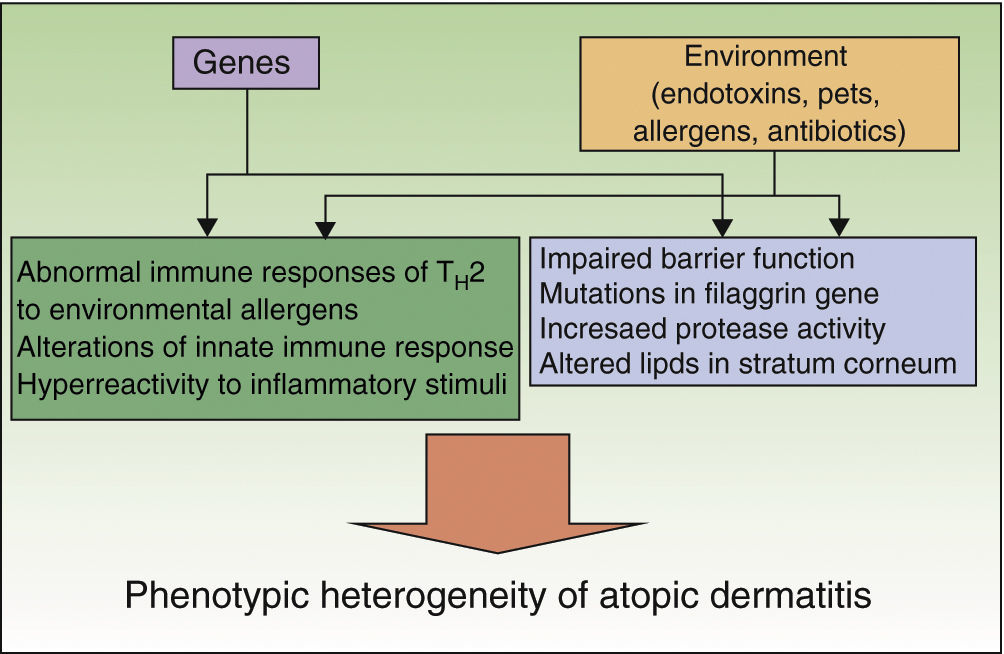
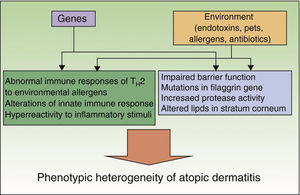
Preventive measures should be based on appropriate knowledge of the etiology and pathogenesis of a disease, which is a limitation in AD given that both are unknown. A series of genetic factors, in particular loss-of-function mutations in the filaggrin gene, interact with environmental factors to produce the various clinical manifestations of AD (Fig. 1).4,5 These genetic factors themselves are insufficient to account for the increased prevalence of AD, as shown by the fact that immigrants from developing countries acquire the prevalence of their host country or the rural/urban difference within a single country.6 Therefore, a series of environmental factors favor the development of an atopic phenotype, whereas others protect against this phenotype. These factors act mainly at very early stages of development (last months of pregnancy and first year of life), as shown by the observation that in more than 60% of cases, AD manifests during the first year of life. Preventive measures should focus on this stage.
Primary PreventionExposure to Microbes: Hygiene HypothesisThe hygiene hypothesis posits that reduced exposure to specific microorganisms during key periods of development leads to immunologic modification favoring acquisition or maintenance of an atopic phenotype.7 It is important to highlight the role of intestinal flora—at least 60% of lymphocytes are in the digestive tract—which is essential for the development of sensitization and immune tolerance.
Probiotics and PrebioticsIn utero, the intestine is sterile, although it quickly becomes colonized after delivery. Intestinal flora is less diverse in children with AD, with reduced presence of Bifidobacterium species and increased presence of Staphylococcus aureus.8 We might ask whether addition of a probiotic or prebiotic could modify intestinal flora by modulating reactivation of the immune system to prevent AD. The relevant literature is abundant, with contradictory findings and considerable heterogeneity between studies in terms of factors such as diagnostic criteria, methodology, strains, and dose. Therefore, it is very difficult to compare studies and the conclusions from meta-analyses. The fact that many authors and the World Allergy Organization feel that more studies are necessary means that it is too early to make appropriate recommendations.9–11 Nevertheless, research should continue in this direction. Special mention should be made of a recent paper,12 in which 26 randomized studies were reviewed (level of evidence, 1a). The authors concluded that probiotics are useful for prevention of AD, but only if they are administered sequentially during the last months of pregnancy and the first months of life. They would prove useless if administered only after birth.
EndotoxinsSeveral epidemiological studies have established an association between specific situations and protection against AD. As mentioned above, these situations are characterized by exposure—especially during pregnancy and the first year of life—to specific microorganisms that can reduce the risk of AD by modifying the immune system. The reduced risk could be due to endotoxins, a group of lipopolysaccharides present in the walls of gram-negative bacteria.
Farm Animals and Unpasteurized MilkChildren who live on farms are thought to have a lower risk of AD, although there is no evidence that merely living on a farm exercises a protective effect.5 The reasons underlying this possibility could be the effect of unpasteurized milk (rich in lactobacilli) and contact with farm animals, especially if exposure is both before and after birth. This protective effect has even been observed in persons from an urban environment who drink unpasteurized milk (level of evidence, 3b).13
PetsThe presence of pets during the first year of life could protect against AD. Several studies show a uniform protective effect with dogs (level of evidence, 2a)14; however, this effect is less clear with cats, and findings are contradictory.15 The risk is greater in children with mutations in the filaggrin gene, suggesting that alterations in the barrier function could facilitate sensitization to cat dander and development of AD.16,17
Helminth InfectionSome articles showed an inverse association between AD and helminth infection, thus explaining, at least in part, differences between developed and developing countries and rural and urban areas (level of evidence, 3b).18,19 Greater evidence for this association is found in various interventional studies, which show an increased risk in pregnant women taking antihelminth therapy in endemic areas (level of evidence, 1b).20 The immunologic basis for this protective effect is not clear, although it could result from an anti-inflammatory effect caused by increased production of interleukin (IL) 10.21
Infections and VaccinesThe several studies that examine infections (viral and bacterial) occurring before birth or during infancy report contradictory results. Some found a slight increase in the risk of AD22 and others a protective effect.23 Schmitt et al.24 observed no association and linked the possible increased risk to more frequent use of antibiotics.
The same is true with vaccines. Whereas some studies suggest a slight increase in risk, more modern studies found no association with AD.
AntibioticsAlthough it can be difficult to discriminate between the use of antibiotics and the infection they are prescribed for, several studies have associated antibiotic therapy with an increased risk of AD. Tsakok et al.25 recently performed a systematic review of 20 studies and found a positive association between antibiotic therapy and the subsequent risk of developing AD, especially if the antibiotics are broad-spectrum. This association was maintained both in the 7 cross-sectional studies and in the 13 longitudinal studies analyzed. The risk was present when therapy was administered both before and after birth. In addition, the risk was cumulative, that is, the more cycles of antibiotics taken, the greater the risk of developing AD. Once again, this increased risk is thought to be due to an alteration in the microflora, which would in turn lead to an alteration in the immune system. Thus, until more robust evidence becomes available, it seems reasonable to recommend that antibiotics, especially broad-spectrum antibiotics, be prescribed with caution (level of evidence, 2a and 3a).
Dietary ModificationsThe recommendations and actions reported in the literature are as follows:
- -
Modification of maternal diet, with a reduction in the so-called allergenic foods, ω-3 supplements, fatty acids, and vitamin D
- -
Replacement of infant formulas with hydrolyzed formulas, soy milk, mineral supplements, and vitamin supplements (eg, vitamin D)
- -
Promotion of breastfeeding
Again, study results are controversial and contradictory, in part owing to very heterogeneous designs. Research in this field is ongoing. It is no surprise that at visits, patients—or parents—ask us which foods should be avoided or if taking a supplement would help to improve the patient's symptoms. In accordance with the information provided above, and until further trials have been performed, no routine recommendations can be made, as demonstrated by meta-analyses (with their inherent bias).9,26
BreastfeedingBreastfeeding has traditionally been considered a major barrier against AD and allergy. In fact, the World Health Organization recommends breastfeeding as the only source of food during the first 6 months. Nevertheless, recent studies and various meta-analyses have shown that this approach could be erroneous, as far as reduction in the frequency of AD and allergy is concerned. Data from the phase II International Study of Asthma and Allergies in Childhood (ISAAC), which includes more than 51 000 children, found no evidence that breastfeeding alone is capable of preventing AD.27 Similarly, a meta-analysis of 27 prospective studies found no protection against AD.28 Breastfeeding is considered the most complete source of food because of its nutritional properties and effects on the immune system. These studies do not change this notion but merely point out that breastfeeding is not useful for preventing atopy and that this should be taken into account when informing patients (level of evidence, 2a). Once again, the reason could be the greater frequency of intestinal infection and colonization with artificial milk; however, immunologic mechanisms could also be involved. A deeply rooted idea in our setting is that late introduction of solid foods can prevent allergy. However, several studies show that early introduction of solid foods, unlike strict allergen avoidance, could be more likely to induce immune tolerance than sensitization.29,30 The several clinical trials that are under way to verify the effect of breastfeeding only compared with breastfeeding and early introduction of solid foods could shed some light on this issue.31
House Dust MitesA significant percentage of patients with AD are sensitized to house dust mites, and some experience outbreaks after exposure.32 Nevertheless, one systematic review found no evidence of improvement when house dust levels were reduced.33 Some studies even found a paradoxical increase in the risk of AD in families who avoided the allergen, much in the same way as when endotoxins are avoided.34,35 Therefore, in practical terms, strict avoidance of house dust mites should not be recommended as a strategy for prevention or treatment, since it is difficult, impractical, and, possibly, erroneous.
Secondary PreventionSecondary prevention measures are aimed at reducing the number and intensity of outbreaks and preventing or diminishing the atopic march. These strategies could have an effect on each other. Furthermore, secondary prevention measures are occasionally involved in primary prevention strategies, since genetic and environmental risk factors are present before and after the disease develops.
Modification of the Atopic MarchThe atopic march is the natural history of atopic manifestations, of which AD is usually the first, followed by food allergy, asthma, and allergic rhinitis. This development is a real phenomenon, although there is some debate as to whether it represents a causal relationship or concurrent manifestations of an atopic phenotype that shares environmental and genetic risk factors.36,37 Since the discovery of the filaggrin gene and its loss-of-function mutation as a risk factor for AD, the loss of the barrier function of the epidermis has become increasingly relevant. The filaggrin mutation is associated with asthma in patients with AD, although not in those with mutations who do not have AD38,39; hence the potential importance of the barrier function of the stratum corneum in sensitization to allergens. The association between severity of AD and more marked alteration of the barrier function and a higher percentage of subsequent sensitizations points to a causal mechanism.37,40–42 It has been postulated that an epidermis with a faulty barrier function enables passage of microorganisms and allergens, leading to epicutaneous sensitization that triggers the atopic march.36,39,43
If we assume that the atopic march is causal and that it is due, at least in part, to a defective skin barrier, then it could be avoided or at least diminished if we could restore the skin barrier. How to achieve this is a key issue.
Restoration of the Defective Skin BarrierThe 2 basic assets in this area are emollients and control of inflammation. In AD, inflammation produces a series of cytokines that can exercise a negative effect on the expression of filaggrin and the synthesis of specific lipids present in the stratum corneum, thus leading to greater transepidermal elimination of water and more marked loss of barrier function.44–46 Therefore, when restoring the epidermal barrier, it is important to control outbreaks.
Reduction of the Intensity/Severity of OutbreaksAD involves subclinical inflammation, with histologic and immunologic abnormalities in the unaffected skin of patients with the disease.47 Several studies report a reduction in the number and intensity of outbreaks when subclinical inflammation is treated with topical tacrolimus administered over 2-3 days per week in both adults and children (level of evidence, 1b).48,49 This so-called proactive treatment is not restricted to calcineurin inhibitors; it can also be administered via topical corticosteroids twice weekly (level of evidence, 1b).50,51 The most appropriate regimen in terms of clinical and cost-effectiveness criteria remains to be determined; however, this is not the object of the present review. Very few studies analyze modification of respiratory symptoms using these treatments. Two studies report on the same group of patients (moderate-severe AD) treated with tacrolimus cream for 1 year for minimal symptoms and up to 1 week after the outbreak was resolved.52,53 The patients were evaluated at 4 and 10 years. The authors found that the severity of the skin manifestations had decreased and that the respiratory symptoms had improved. Nevertheless, the study is subject to bias, since it is open-label with no control group, thus preventing us from drawing clear conclusions (level of evidence, 4). In addition, the study population comprised adult patients with AD and established respiratory symptoms. Ideally, children should be analyzed to evaluate the effect of these early treatments on subsequent manifestations of atopic disease. A similarly designed trial was performed with topical pimecrolimus; however, unfortunately, the study ended early owing to loss of patients, and no conclusions could be drawn.54 This trial coincided with the alert from the United States Food and Drug Administration on the risk of neoplasm associated with calcineurin inhibitors. The alert may account for the loss of patients and the early termination of the study.
EmollientsMoisturizing with emollients has traditionally been the cornerstone of management of AD.55 In addition to reducing the loss of water through the skin, emollients relieve itching and increase the efficacy of and reduce the need for topical corticosteroids.56,57 They are increasingly used as a result of the discovery of the role of filaggrin and other proteins in the stratum corneum and advances in our knowledge of the lipid composition of this layer. Just as inflammation leads to a more intense alteration of barrier function, correct hydration can reduce the inflammation observed in the dry skin of patients with AD.40,58,59
Emollients can be classed as nonphysiologic (eg, petrolatum and lanolin), that is, they are not present naturally in the skin, or physiologic. Physiologic emollients are composed of lipids present in the stratum corneum and have proven to be defective in patients with AD. They include ceramides, cholesterol, and free fatty acids. Emollients can penetrate the stratum corneum, are taken up by keratinocytes, processed in lamellar bodies, and resecreted to the stratum corneum more naturally. However, our knowledge is theory-based, since the few studies available were performed with low numbers of patients and short follow-up periods. Furthermore, almost no results are available from randomized controlled trials on the various types of emollients, although several studies are ongoing.
Emollients used in primary preventionCurrent efforts to develop emollients are focused on restoration of barrier function and improvement in the symptoms of AD. Very few studies examine primary prevention. Simpson et al.60 studied 22 neonates from families at a high risk of developing AD who used an emollient (oil-in-water) from birth. Clear conclusions cannot be drawn, since the study did not have a control group. However, a comparison with historic controls led the authors to suggest a decrease in incidence (level of evidence, 4). More recently, Inoue et al.61 compared 147 neonates with a control group but found no differences with respect to incidence. They did find differences, however, for severity, which was lower in children treated twice daily with emollients. The authors performed a single measurement at 4 months of age, which is insufficient in a disease such as AD (level of evidence, 3b). In any case, this approach is being investigated in ongoing clinical trials, although more time and more studies are necessary before firm conclusions can be drawn.
Conclusions and ReflectionsIt is impossible to prevent a disease as complex and multifactorial as AD by eliminating a single risk factor. AD requires an integral approach involving various health professionals and public authorities and enabling different prevention strategies to be promoted. Unfortunately, such strategies have not yet been established and cannot be routinely recommended, since there is insufficient evidence (Table 2). However, this does not necessarily mean that these strategies do not work, only that we must perform additional studies, many of which are already under way. The clearest example is the use of probiotics and prebiotics in primary prevention, which has yielded promising results in some clinical trials. Other measures are simply impractical and cannot be applied at the population level. Although helminth infection and the endotoxins of specific bacteria reduce the risk of AD, infecting children or pregnant women with this purpose in mind is unthinkable. Instead, attempts are being made to isolate components of the helminth wall and various bacterial lipopolysaccharides so that they can be administered exogenously. Results are available from murine and human studies, although these remain scarce. Nevertheless, this aspect warrants further analysis.62,63
Strategies for the Prevention of Atopic Dermatitis.
| Primary Prevention |
| Exposure to Microbes |
| Probiotics and prebiotics |
| Endotoxins |
| Pets |
| Unpasteurized milk |
| Reduced use of broad-spectrum antibiotics |
| Immune tolerance |
| Environmental allergens |
| Foods |
| Secondary Prevention |
| Effective control of outbreaks |
| Restoration of the skin barrier |
| Treatment of subclinical inflammation |
| Emollients |
Halting the atopic march is of considerable interest, since a clear temporal relationship and biological plausibility have been observed. Nevertheless, interventional studies confirming causality and the real possibility of halting the disease have yet to be performed. If this progression could be prevented by controlling inflammation (clinical and subclinical) and restoring barrier function, disease course could actually be modified.
Also controversial is the selection of candidates for preventive measures. The high prevalence of AD and its implication in the development of other atopic diseases would justify taking measures in the general population. Nevertheless, as mentioned above, these measures have not been well defined, and many clinical trials have focused on at-risk patients (first-degree relatives of atopic individuals). Furthermore, dermatologists have easy access to these individuals. It is in this specific group that we can establish appropriate measures or, based at least on our knowledge of etiology and pathogenesis and advances in epidemiology, we could inform patients appropriately and avoid unsuitable and erroneous measures and disinformation.
Lastly, genetic studies and new biomarkers will probably enable different subtypes or phenotypes of AD to be established, thus enabling more personalized therapeutic and preventive measures to be developed.
Conflicts of InterestThe author declares that he has no conflicts of interest.
Please cite this article as: Gómez-de la Fuente E. ¿Se puede prevenir la dermatitis atópica?. Actas Dermosifiliogr. 2015;106:278–284.