Few studies have addressed cutaneous recurrence of melanoma. The aim of this retrospective study was to analyze the characteristics and prognostic significance of the different patterns of cutaneous recurrence.
Material and methodsPatients diagnosed with melanoma between 1988 and 2008 at Hospital de Bellvitge, Barcelona, Spain and for whom data were available for at least 2 years of follow-up were included in the study. Local recurrence was defined as melanoma invasion of the skin adjacent to the scar left by excision of the primary tumor, regional metastasis or recurrence as metastasis restricted to the area drained by a regional lymph node station, and distant cutaneous metastasis as metastasis occurring outside this area. The relationship between cutaneous recurrence pattern and age, sex, primary tumor site, tumor subtype, Breslow depth, and ulceration was assessed.
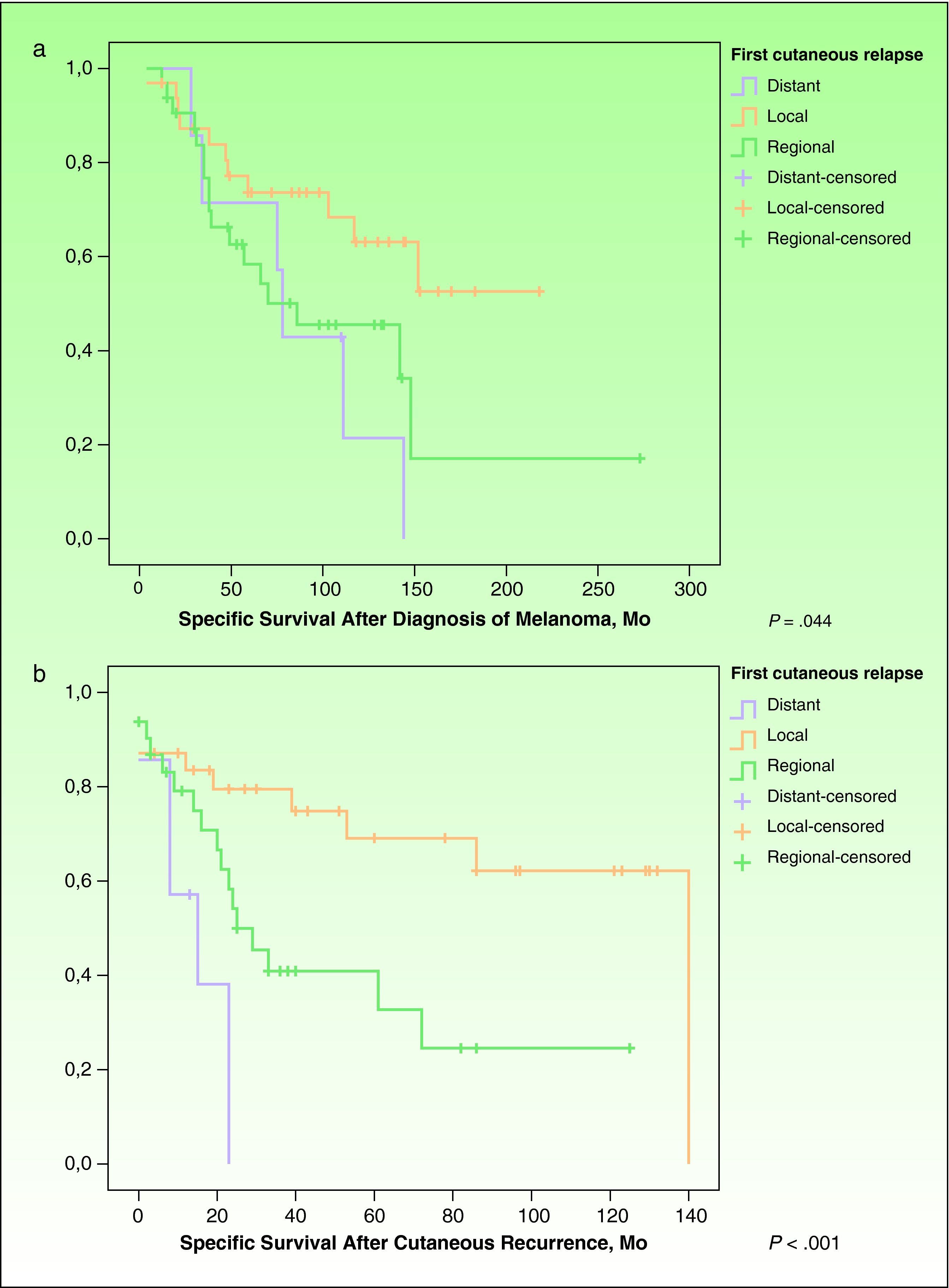
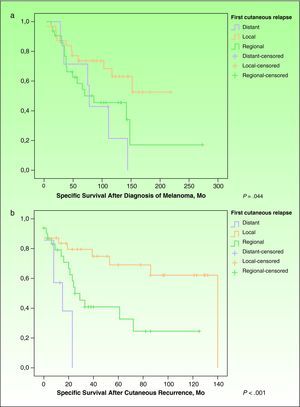
ResultsEighty-five out of 1,080 patients (7.87%) had cutaneous recurrence. In 71 of those patients (83.53%; 27 men and 44 women; mean age, 60.68 years), this was the first indication of melanoma recurrence. Thirty-two patients had local recurrence, 32 regional metastasis, and 7 distant metastasis. Significant differences were observed in survival time from diagnosis of the primary tumor (P=.044) and from diagnosis of cutaneous recurrence (P<.001) according to the type of recurrence.
ConclusionsOur results suggest that the pattern of cutaneous recurrence is prognostically significant and related to the site of the primary tumor given that the majority of local and regional recurrences occurred in primary tumors located on the lower limbs and head.
Existen pocos estudios sobre recidiva cutánea en el melanoma. Nuestro objetivo fue analizar retrospectivamente las características y el significado pronóstico de los distintos patrones de recidiva cutánea del melanoma.
Material y métodosLos pacientes con melanoma diagnosticados entre 1988-2008 en el Hospital de Bellvitge de Barcelona, con más de dos años de seguimiento, fueron incluidos en el estudio. Se consideró recidiva local a la infiltración cutánea por melanoma en continuidad con la cicatriz de exéresis del tumor primario, metástasis o recidiva regional cuando las lesiones se mantenían en el territorio de drenaje linfático regional y metástasis cutánea a distancia cuando se localizaron fuera de este territorio. Se analizó la relación del patrón de recidiva cutánea con edad, sexo, localización del tumor primario, tipo clinicopatológico, profundidad máxima de invasión y ulceración.
ResultadosOchenta y cinco de 1.080 pacientes desarrollaron recidiva cutánea (7,87%). En 71 de 85 pacientes (83,53%) la recidiva cutánea fue la primera evidencia de recidiva (27 varones y 44 mujeres; edad media: 60,68 años). Treinta y dos pacientes presentaron recidiva local, 32 regional y 7 a distancia. Las curvas de supervivencia mostraron diferencias significativas en tiempo de supervivencia específica desde el diagnóstico del melanoma primario (p=0,044) y desde el diagnóstico de la recidiva cutánea (p<0,001).
ConclusionesNuestros resultados sugieren que el patrón de recidiva cutánea tiene significado pronóstico y que está relacionado con la localización del tumor primario, puesto que la mayoría de recidivas locales y regionales se producen en las extremidades inferiores y en la cabeza.
During the last few decades, the incidence of cutaneous melanoma has increased more than that of any other malignant neoplasm.1–3 Interest from the scientific community and the number of publications on melanoma have increased in parallel. Yet, few clinical studies have analyzed cutaneous recurrence patterns in malignant melanoma.4–7 Some studies have included cutaneous metastases in multivariate models, such as the recent study of the American Joint Committee on Cancer (AJCC), in which the presence of regional and distant cutaneous metastases were independent prognostic factors, although the authors did not specifically analyze the cutaneous recurrence pattern or its correlation with other parameters.8
Our objective was to analyze the prognostic significance of the cutaneous recurrence pattern in malignant melanoma and its association with other clinicopathologic characteristics.
Material and MethodsThe study population comprised all patients with malignant cutaneous melanoma (stage I, II, or III) at diagnosis who were disease-free after their initial treatment at Hospital de Bellvitge, Barcelona, Spain between 1988 and 2008 and had a minimum 2 years of follow-up. The institution is a tertiary-level teaching hospital that provides health care to approximately 1 million people. Patients with stage IV melanoma at diagnosis and patients with melanoma of unknown origin were excluded.
Melanomas in situ were removed with a 5-mm margin, melanomas in the vertical growth phase with a maximum depth of < 1mm (Breslow depth) were removed with a 1-cm margin, melanomas measuring 1.01mm to 2mm were removed with 2-cm margins, and melanomas > 2mm deep were removed with 3-cm margins. Until 2000, regional nodes were removed when primary tumors presented a Breslow depth > 1.5mm and were located on the extremities. When the location was the trunk or head, this approach was used only when the primary tumor lay adjacent to the lymph drainage area corresponding to the primary tumor. From 2000 onward, sentinel node biopsy was applied in all melanomas with a Breslow depth > 1mm, and regional nodes were removed in cases of invasion of the sentinel node by the melanoma.
Clinical follow-up was at the dermatology department every 4 months during the first 2 years after diagnosis and every 6 months until 5 years after diagnosis. Follow-up in the case of melanomas with a Breslow depth <1mm was for 5 years; patients were then referred to their area dermatologist for lifetime yearly checkups. Follow-up in the case of melanomas with a Breslow depth > 1mm was for 10 years at the dermatology department; patients were then referred to their area dermatologist for lifetime yearly checkups.
During the first 2 years of follow-up, blood chemistry (including lactate dehydrogenase) was assessed at each visit and a yearly radiograph was taken. Additional tests were performed depending on clinical and laboratory findings.
Retrospective data retrieved from the clinical histories were entered into a digital database to which only the authors of the present article had access. The data collected included sex, age at diagnosis, site of the primary tumor, date of diagnosis, date of cutaneous recurrence, date of most recent checkup, and, where applicable, date of death due to melanoma. The clinical type of recurrence was evaluated in each case according to the following criteria: local recurrence in the presence of invasion of the skin adjacent to the scar left by excision of the primary tumor, with margins considered acceptable according to our protocol; and regional metastasis or regional recurrence when the lesions were restricted to the area drained by a regional lymph node station of the primary tumor (satellitosis in lesions limited to 5cm from the scar left by excision of the primary tumor and in-transit metastasis when at a greater distance). Finally, lesions outside the area drained by a regional lymph node of the primary tumor were classed as distant cutaneous metastasis. The histopathology data collected were clinicopathologic type, maximum Breslow depth, Clark level, and presence of ulceration.
As for statistical analysis, quantitative variables were compared using analysis of variance, and qualitative variables were compared using contingency tables. Given the limited number of cases, the χ2 test could not be applied in many cases, and we were only able to estimate the existence of correlations. Survival curves were analyzed using the Kaplan-Meier method. The log-rank test was used to detect significant differences between the survival curves. The statistical analysis was performed using SPSS 13.0 for Windows. Statistical significance was set at P<.05. The low number of cases prevented us from performing a multivariate analysis.
ResultsA total of 1080 patients with cutaneous malignant melanoma who were disease-free after the initial treatment completed the minimum 2 years of follow-up. Cutaneous recurrence of melanoma was detected in 85 (7.87%) patients. In 71 (83.53%), cutaneous recurrence was the first evidence of recurrence of melanoma after the initial treatment. This group comprised 27 men and 44 women (mean age, 60.68 years). The first recurrence was local in 32 patients, regional cutaneous metastasis in 32, and distant cutaneous metastasis in 7.
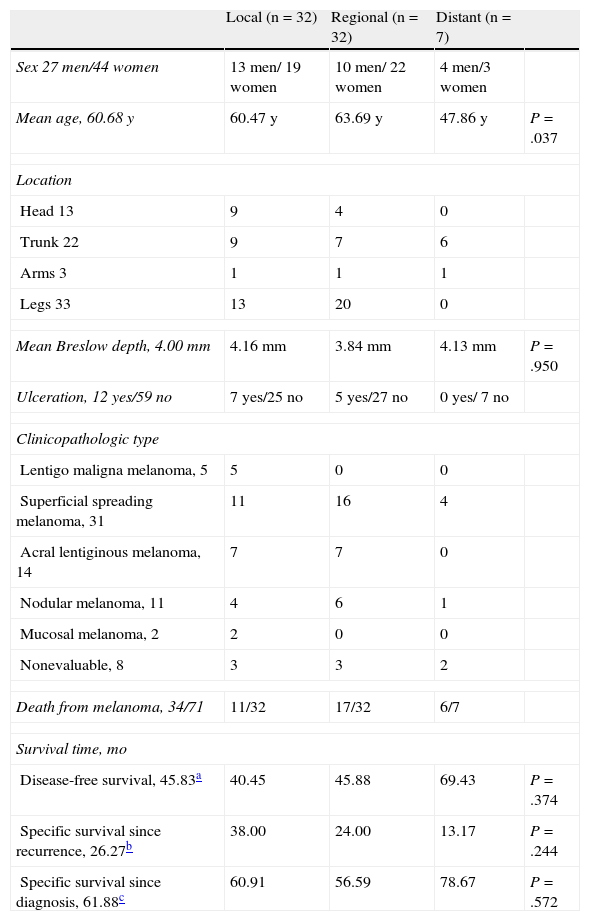
For each type of recurrence, Table 1 shows the following data: sex, mean age, site of the primary tumor, mean maximum Breslow depth, presence or absence of ulceration, clinicopathologic type, and number of patients who died of the melanoma in each category, as well as mean disease-free survival, mean cause-specific survival after cutaneous recurrence, and mean cause-specific survival after diagnosis of melanoma.
Clinicopathological Variables Analyzed in Patients With Cutaneous Recurrence as the First Evidence of Recurrence of Melanoma After the Initial Treatment (71 Patients).
| Local (n=32) | Regional (n=32) | Distant (n=7) | ||
| Sex 27 men/44 women | 13 men/ 19 women | 10 men/ 22 women | 4 men/3 women | |
| Mean age, 60.68 y | 60.47 y | 63.69 y | 47.86 y | P=.037 |
| Location | ||||
| Head 13 | 9 | 4 | 0 | |
| Trunk 22 | 9 | 7 | 6 | |
| Arms 3 | 1 | 1 | 1 | |
| Legs 33 | 13 | 20 | 0 | |
| Mean Breslow depth, 4.00 mm | 4.16 mm | 3.84 mm | 4.13 mm | P=.950 |
| Ulceration, 12 yes/59 no | 7 yes/25 no | 5 yes/27 no | 0 yes/ 7 no | |
| Clinicopathologic type | ||||
| Lentigo maligna melanoma, 5 | 5 | 0 | 0 | |
| Superficial spreading melanoma, 31 | 11 | 16 | 4 | |
| Acral lentiginous melanoma, 14 | 7 | 7 | 0 | |
| Nodular melanoma, 11 | 4 | 6 | 1 | |
| Mucosal melanoma, 2 | 2 | 0 | 0 | |
| Nonevaluable, 8 | 3 | 3 | 2 | |
| Death from melanoma, 34/71 | 11/32 | 17/32 | 6/7 | |
| Survival time, mo | ||||
| Disease-free survival, 45.83a | 40.45 | 45.88 | 69.43 | P=.374 |
| Specific survival since recurrence, 26.27b | 38.00 | 24.00 | 13.17 | P=.244 |
| Specific survival since diagnosis, 61.88c | 60.91 | 56.59 | 78.67 | P=.572 |
The association between recurrence patterns and qualitative variables was compared using contingency tables; although statistical tests could not be applied, a marked tendency was observed towards an association between the cutaneous recurrence pattern and the site of the primary tumor and, to a lesser extent, sex. Certain clinicopathological types of melanoma also tended to be associated with specific cutaneous recurrence patterns (lentigo maligna melanoma with local recurrence) (Table 1).
Analysis of variance revealed significant differences in age between the 3 categories of cutaneous recurrence (P=.037), but not in Breslow depth (Table 1). Analysis of variance did not reveal significant differences for disease-free survival, cause-specific survival after cutaneous recurrence, and cause-specific survival after diagnosis of the primary tumor (Table 1).
However, Kaplan-Meier survival curves for each type of recurrence revealed significant differences in survival time since the diagnosis of melanoma (P=.044) (Fig. 1A) and in survival time since the diagnosis of cutaneous recurrence (P=.001) (Fig. 1B).
A, Specific survival after diagnosis of the primary tumor. Survival curves for each type of cutaneous recurrence from the diagnosis of the primary tumor until death from melanoma (Kaplan-Meier, P=.044). B, Specific survival from the time of the cutaneous recurrence. Survival curves for each type of relapse from diagnosis of cutaneous relapse until death from melanoma (Kaplan-Meier, P=.001).
Patients with local and regional recurrence were treated with surgery where possible. When there were several lesions or when lesions reappeared after excision, patients were treated with radiotherapy. Two patients with regional cutaneous recurrence of multiple small lesions were treated with topical imiquimod; however, response was partial, and the patients were subsequently treated with radiotherapy. In patients with distant cutaneous metastasis, lesions were excised if there were few of them. However, as most patients developed visceral metastasis after a few months, they received chemotherapy (with dacarbazine in most cases).
DiscussionAccording to the literature, cutaneous metastasis is relatively common in the natural history of melanoma, occurring in 2% to 20% of cases depending on the series.4,7,9,10 However, little is known about the risk factors involved in the development of different patterns of cutaneous recurrence (local, regional metastasis, or distant metastasis) or their prognostic significance.4 Some studies distinguish between local, regional, and distant recurrence, although others do not distinguish between local and regional recurrence or focus solely on one of the two. In addition, the conclusions are not consistent.4–6 According to some studies, patients with different cutaneous recurrence patterns do not present significant differences in survival.7 In contrast, the prognostic significance of cutaneous metastasis was recently analyzed in an AJCC multivariate study by Balch et al,8 in which regional metastases were category N2c and distant cutaneous metastases were category M1a and, therefore, have prognostic significance. However, the study was wide-ranging and did not analyze the prognostic significance of local recurrence or the correlation between the cutaneous recurrence pattern and other parameters.
Discrepancies can also be found with respect to the number of patients in whom cutaneous recurrence is the first sign of progression of melanoma. For example, in a recent study, cutaneous recurrence was the first sign of tumor progression in only 56.3%.4 However, in our study, when melanoma spread to the skin, it was often the first sign of melanoma recurrence (83.53%).
Savoia et al4 recently found that the cutaneous recurrence pattern was closely related to the site of the primary tumor. We also found that the site of the primary tumor affected the pattern of cutaneous dissemination (Table 1). In fact, patients with melanoma on the lower extremities and on the head only developed local and regional cutaneous recurrence. Patients with melanoma on the trunk presented local, regional, and distant recurrence with a similar frequency, whereas cutaneous relapses were exceptional in tumors located on the upper extremities (only 3 cases) (Table 1). It is noteworthy that most distant cutaneous metastases occurred in melanomas on the trunk (6 of the 7 patients with distant cutaneous metastases had a primary tumor on the trunk) and that 20 of the 32 patients with regional cutaneous metastases had a primary tumor on the lower extremities. The reason why regional metastases are usually observed on the lower limbs is unknown. According to the literature, locoregional metastases are favored by embolization of dermal lymph vessels between the primary tumor and regional lymph nodes. In addition, a correlation has been detected between lymphatic invasion and subsequent locoregional recurrence.11 Some authors consider that lymphatic stasis after node removal is an additional risk factor and argue that most of their patients with regional metastasis have the primary tumor on the lower limbs, where lymphedema is common after node removal.4 However, lymphedema can also appear on the arms after axillary node removal, and, in our study, we only observed 1 case of regional cutaneous metastasis on the arms, thus contradicting the above argument.
The analysis of the association between the cutaneous recurrence pattern and the other variables studied highlights that local and regional recurrence were more common in women, possibly because of a greater frequency of primary tumors on the lower limbs among women (83% of our patients with melanoma on the lower limbs were women). It is also noteworthy that the mean age of our patients with distant metastasis was significantly lower (47.86 years) than that of patients with local recurrence (60.47 years) and regional recurrence (63.69 years). We found that the cutaneous recurrence pattern was not related to Breslow depth, as we did not detect significant differences between the 3 patterns. Nor did we detect significant differences between the cutaneous recurrence patterns for the remaining clinicopathologic characteristics analyzed (Table 1).
As for survival, our patients with local recurrence of melanoma in situ progressed favorably with local surgery. Of the remaining patients with local recurrence of invasive melanoma, 11 of 30 died of melanoma. In the group with regional cutaneous recurrence, 17 of 32 died. Six of 7 patients with distant cutaneous metastasis died. Although analysis of variance revealed no significant differences, patients with local recurrence and patients with regional recurrence had a shorter recurrence-free period once the primary tumor was removed (40.45 and 45.88 months, respectively), compared with cases of distant cutaneous metastasis (69.43 months) (Table 1). However, once the first cutaneous recurrence developed, time to death from melanoma was longer for patients with local and regional recurrence (38.00 and 24.00 months, respectively) than for patients with distant cutaneous metastasis (13.17 months) (Table 1 and Fig. 1B). Kaplan-Meier analysis revealed that survival was significantly impacted by the cutaneous recurrence pattern: significant differences were observed for cause-specific survival after diagnosis of the primary tumor and cause-specific survival after diagnosis of the cutaneous recurrence (Fig. 1A and B). These results are consistent with the prognostic value observed for distant and regional cutaneous relapse in the study by Balch et al,8 on which the current prognostic classification of the AJCC is based.
Our results suggest that differences in prognosis between the various types of cutaneous relapse should be taken into account in clinical management and in the choice of possible therapeutic options. Given the relatively favorable prognosis of patients with local or regional cutaneous recurrence, first-choice approaches were local but barely aggressive treatments such as surgery, radiotherapy, perfusion, and electrochemotherapy, whereas patients with distant cutaneous metastasis and a short period until development of visceral metastasis (only 13.17 months) could benefit from more aggressive systemic treatment.4,12 Follow-up of the latter group should be more meticulous owing to the frequent and rapid onset of visceral metastasis.
Our study is limited by its retrospective design and the small sample size, which prevented us from performing a multivariate analysis. Consequently, confounders could be present. The χ2 test could not be applied in many cases, and we were only able to estimate the existence of correlations.
Our results suggest that the cutaneous recurrence pattern has prognostic significance and that it depends on the location of the primary tumor, given that most local and regional recurrences occur in melanomas located on the lower limbs and head.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Marcoval J, et al. Análisis descriptivo de los patrones de recidiva cutánea en los pacientes con melanoma. Actas Dermosifiliogr. 2011;102:791-796.