To the Editor:
The term calciphylaxis has been used since the 1960s1 to describe skin ulceration secondary to vascular calcifications in patients with terminal renal failure and secondary hyperparathyroidism.2 However, nonuremic causes have been reported and the mechanism of cutaneous vascular calcification has now been investigated more extensively.3 The condition is currently considered a complex multifactorial process and not a simple deposition. Daudén et al.4 proposed a new classification of these processes, using cutaneous vascular calcification (CVC) as a general term, and we are in agreement with this terminology.
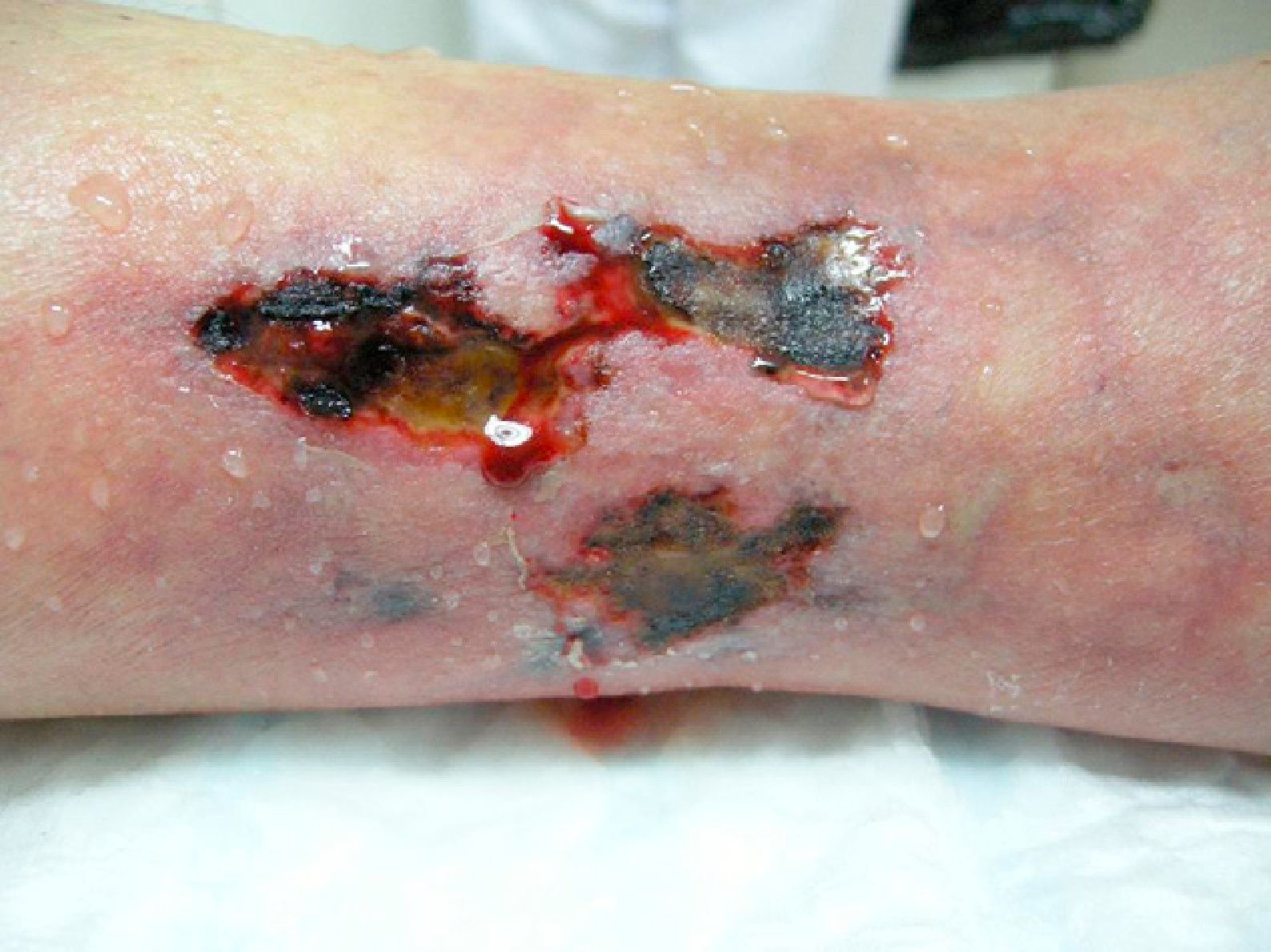
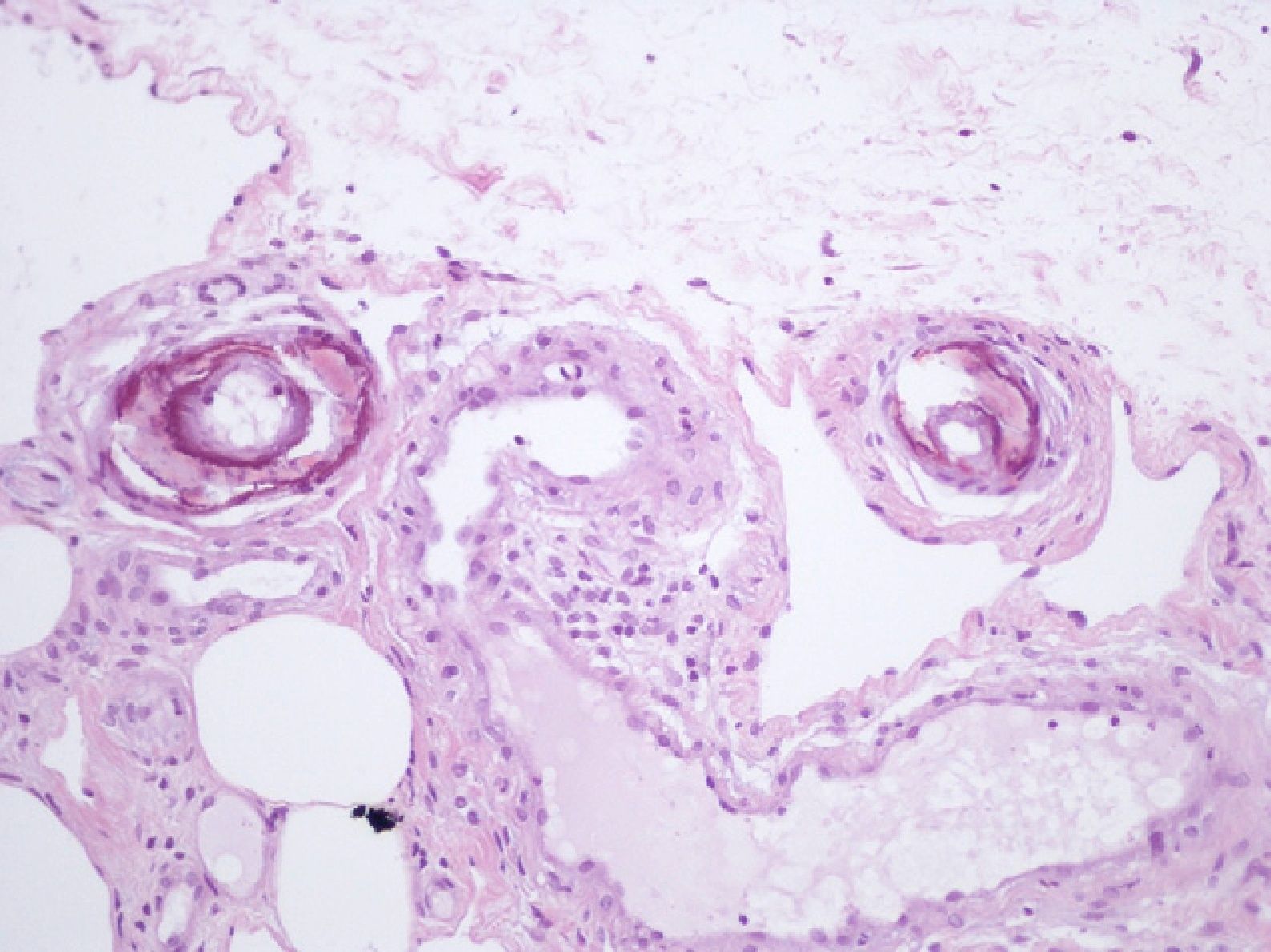
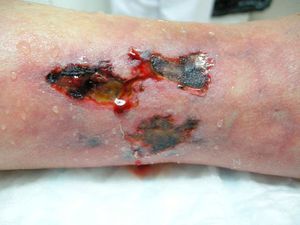
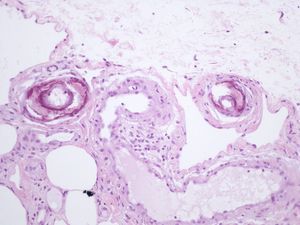
We report the case of an 80-year-old woman with a history of severe refractory osteoporosis, hypertension, obesity, atrial fibrillation, and polymyalgia rheumatica treated with corticosteroids. As her osteoporosis was resistant to the usual treatments, she was prescribed the human recombinant peptide teriparatide. The active fragment is a 34 amino-acid sequence of parathyroid hormone (rhPTH). The agent is administered subcutaneously at a dose of 20μg every 24hours. Two months after starting treatment, the patient developed painful necrotic ulcers on the legs on areas of skin with a livedoid appearance (Fig. 1). The echo-Doppler study, renal function tests, calcium-phosphate product, and autoimmune studies were all normal. Skin biopsy (Fig. 2) showed ulceration and necrosis of the epidermis, dilatation of the dermal vessels, and circumferential calcification in walls of small arteries at the dermal-epidermal junction. Immunofluorescence was negative. These findings were consistent with calciphylaxis, but renal failure was not present. However, on administration of teriparatide, which acts like endogenous parathyroid hormone (PTH), a pharmacological state of hyperparathyroidism had been induced. The drug was withdrawn and, at 3 weeks after discontinuation, the patient's lesions improved progressively. She died from progression of her heart failure 6 months after onset of her skin condition. At the time of death, she was free of skin lesions (Fig. 3).
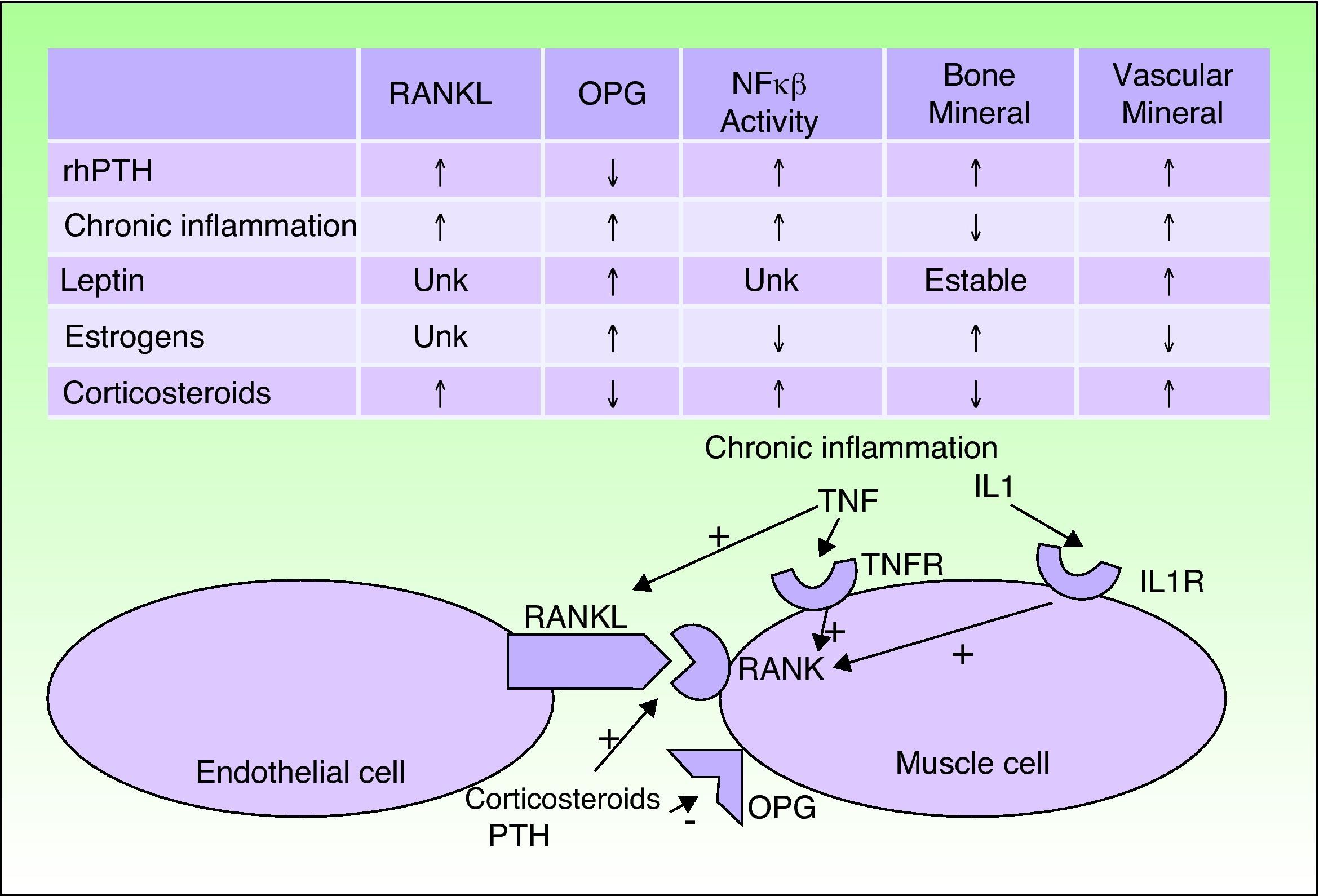
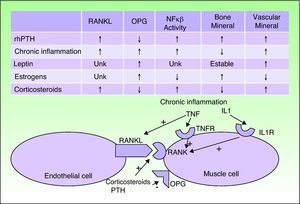
CVC usually occurs in patients with certain predisposing factors, such as obesity, chronic inflammation, corticosteroid treatment, and menopause, and also in individuals with abnormal calcium phosphate metabolism and PTH levels. In recent years, the pathogenic relationships between these factors have been determined. The vascular endothelial cells are seen to adopt an osteogenic phenotype in these patients. Both vascular endothelial cells and osteoblasts, osteoclasts, and vascular smooth muscle cells can express the receptor activator of nuclear factor κ B (RANK) and its ligand RANKL on their membranes; when RANK is activated, the activity of nuclear factor κB (NFκB), a transcription factor, is increased. Osteoprotegerin is a soluble RANKL antagonist produced by the same types of cells. The risk factors mentioned above and PTH interact with this signaling chain.5–7
When the activity of NFκB increases, calcium is deposited in the vessels but lost from bone. Chronic inflammatory disorders, corticosteroids, and PTH activate RANK and RANKL. Corticosteroids and PTH also decrease osteoprotegerin and so further increase the activity of NFκB. Estrogens exercise a protective effect against osteoporosis and CVC by increasing osteoprotegerin expression. After the menopause, this protection is lost. A similar effect is seen for leptin, which is increased in obesity. However, hyperleptinemia in patients with chronic inflammation seems to stimulate CVC (Fig. 3).
Teriparatide acts in the same way as endogenous PTH and has been shown to be effective in refractory osteoporosis.8 In clinical trials, calcium and phosphorus abnormalities were only detected in the first few hours after administration9; laboratory tests only detect intact PTH, and so levels of this hormone were likewise not elevated. Our hypothesis is that our patient had several risk factors such as obesity, menopause, chronic inflammation, and corticosteroid treatment, and administration of rhPTH caused an imbalance in the NFκB signaling cascade, and this triggered CVC. The time course, the improvement after withdrawal of the drug, and the mechanisms of action of the drug would seem to support this hypothesis.
Please cite this article as: Leis-Dosil VM, et al. Calcificaciones vasculares cutáneas secundarias al tratamiento con teriparatida. Actas Dermosifiliogr.2013;104:87-8.