Cutaneous leiomyosarcoma is a malignant neoplasm derived from smooth muscle cells. Its low incidence hampers the development of specific protocols for diagnosis and treatment.
ObjectivesTo describe the clinical and histopathologic characteristics of a series of primary and secondary cutaneous leiomyosarcomas and to determine how these characteristics correlate with prognosis.
Material and methodsWe performed an observational, descriptive, retrospective study based on 17 cutaneous leiomyosarcomas in 12 patients diagnosed between January 1, 2000 and December 31, 2015. We recorded demographic data, clinical and histopathologic characteristics, outcome, and response to treatment.
ResultsWe included 5 men and 7 women, all aged more than 50 years at diagnosis. There were 4 cutaneous leiomyosarcomas (23%) in 4 patients, 2 subcutaneous leiomyosarcomas (11.5%) in 2 patients, and 11 skin metastases of leiomyosarcoma (65%) in 6 patients. The most frequently affected sites were the scalp (41%), lower limbs (17%), and trunk (17%). During follow-up, 50% of the cutaneous leiomyosarcomas recurred, 50% of the subcutaneous leiomyosarcomas presented distant metastases, and 83% of the patients with skin metastases of leiomyosarcoma died of their disease.
LimitationsOurs was a retrospective review of a small case series at a single center.
ConclusionsCutaneous leiomyosarcoma is an uncommon malignant neoplasm. Our approach to diagnosis and therapy must take into account the marked heterogeneity in the prognosis of the various subtypes.
El leiomiosarcoma de piel es una neoplasia maligna de estirpe muscular cuya baja incidencia dificulta el desarrollo de protocolos específicos de diagnóstico y manejo terapéutico.
ObjetivosDescribir las características clínicas e histopatológicas de una serie de leiomiosarcomas cutáneos primarios y secundarios, junto con su correlación pronóstica.
Material y métodosSe realizó un estudio retrospectivo, descriptivo y observacional. Se seleccionaron 17 casos de leiomiosarcoma cutáneo en 12 pacientes, diagnosticados entre el 1 de enero de 2000 y el 31 de diciembre de 2015. Se recogieron sus datos demográficos, características clínicas e histopatológicas, evolución y respuesta al tratamiento.
ResultadosSe reclutaron 5 varones y 7 mujeres, todos ellos mayores de 50 años al diagnóstico. Se recogieron 4 leiomiosarcomas dérmicos (4/17, 23%) en 4 pacientes, 2 leiomiosarcomas hipodérmicos (2/17, 11,5%) en 2 pacientes, y 11 metástasis cutáneas de leiomiosarcoma (11/17, 65%) en 6 pacientes. Las localizaciones más frecuentes fueron cuero cabelludo (7/17, 41%), miembros inferiores (3/17, 17%) y tronco (3/17, 17%). Durante el seguimiento, un 50% de leiomiosarcomas dérmicos recidivaron, un 50% de leiomiosarcomas hipodérmicos presentaron metástasis a distancia y 5/6 pacientes con metástasis cutáneas de leiomiosarcoma (83%) fallecieron a causa de su enfermedad.
LimitacionesEste estudio es una revisión retrospectiva de una serie de casos de tamaño limitado en un centro único.
ConclusionesEl leiomiosarcoma cutáneo es una neoplasia maligna poco frecuente. A la hora de adoptar una actitud diagnóstico-terapéutica en estos pacientes debemos tener en cuenta la marcada heterogeneidad pronóstica entre sus diferentes subtipos.
Skin leiomyosarcomas are rare malignant neoplasms that derive from smooth muscle tissue. They account for around 2% to 3% of all soft-tissue sarcomas in skin and 0.04% of all neoplasms. They are currently thought to arise de novo from smooth muscle, even though there have been reports of tumors that derived from leiomyomas.1
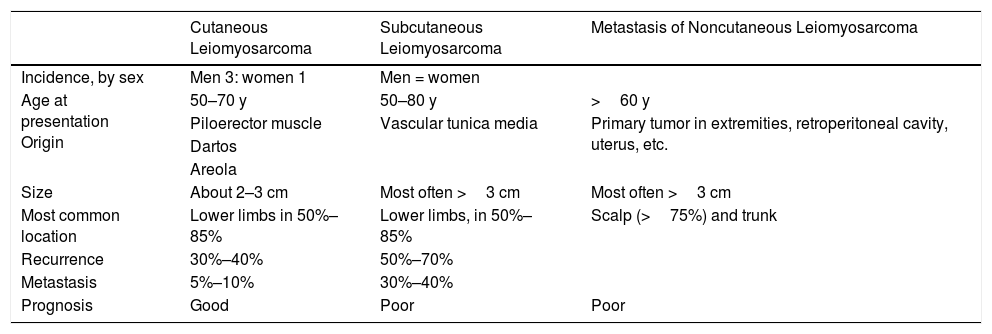
Leiomyosarcomas have traditionally been grouped in 3 large subtypes according to clinical and pathologic features that have prognostic implications: 1) cutaneous, or dermal forms; 2) subcutaneous, or hypodermal forms; and 3) secondary metastatic forms. The most important clinical and prognostic differences between the classes are summarized in Table 1.2,3
Characteristics of Cutaneous, Subcutaneous, and Metastatic Forms of Leiomyosarcoma.
| Cutaneous Leiomyosarcoma | Subcutaneous Leiomyosarcoma | Metastasis of Noncutaneous Leiomyosarcoma | |
|---|---|---|---|
| Incidence, by sex | Men 3: women 1 | Men = women | |
| Age at presentation Origin | 50–70 y | 50–80 y | >60 y |
| Piloerector muscle | Vascular tunica media | Primary tumor in extremities, retroperitoneal cavity, uterus, etc. | |
| Dartos | |||
| Areola | |||
| Size | About 2–3 cm | Most often >3 cm | Most often >3 cm |
| Most common location | Lower limbs in 50%–85% | Lower limbs, in 50%–85% | Scalp (>75%) and trunk |
| Recurrence | 30%–40% | 50%–70% | |
| Metastasis | 5%–10% | 30%–40% | |
| Prognosis | Good | Poor | Poor |
The origin of cutaneous leiomyosarcomas seems to be the pilorector muscle in the dermis. However, there have been reports of cases in which the tumor developed in the dartos, or its equivalent muscle in the vulva, or in the areola. This form is not usually aggressive but tends to recur locally after surgical excision with narrow margins. The risk of distant metastasis is considered low (5%–10%), and these tumors have therefore been called “atypical intradermal smooth muscle neoplasms.”4 Subcutaneous leiomyosarcomas arise in the smooth muscle fibers of the tunica media of arteries and veins. Unlike more superficial, or dermal forms, these leiomyosarcomas have high rates of local–regional recurrence and metastasis (30%–40%), presenting a poor prognosis. Finally, metastatic leiomyosarcoma of the skin is associated with a particularly poor prognosis. Metastasis usually derives from a primary leiomyosarcoma in the retroperitoneum, the uterus, or the subfascial soft tissue of the extremities.5–7Even though the World Health Organization's 2005 taxonomy distinguishes true cutaneous from subcutaneous forms8 (the latter of which are included among soft-tissue tumors9), this distinction can become complicated in clinical practice because of overlap of histopathologic features with prognostic implications.10 In addition, metastases of noncutaneous leiomyosarcomas to the skin simulate the features of primary subcutaneous leiomyosarcomas, a situation that makes metastasis a diagnosis of exclusion.6
Because prognosis is difficult in some cases of leiomyosarcoma in the skin, we analyzed features of cases diagnosed in our hospital in the past 15 years. We included all leiomyosarcomas regardless of origin of the neoplasm, that is, both primary tumors and metastatic disease.
Material and MethodsThis retrospective, descriptive, observational study included data from adults who received a histopathologic diagnosis of leiomyosarcoma between January 1, 2000, and December 31, 2015. Cases were compiled from the skin and soft-tissue sarcoma registry of our hospital's pathology department. We included skin leiomyosarcomas whether they were primary or metastatic. We excluded subfascial and visceral tumors and cases for which the clinical history was unavailable or incomplete.
We collected the following data from the records of selected patients: age at presentation, sex, year diagnosed, clinical presentation (lesion type, number, size, and location), latency time (from diagnosis of the primary tumor until detection of metastatic disease in skin), length of follow-up, clinical course, and response to treatments (disease-free, recurrence, or death). Histologic features recorded were histologic form (cutaneous or subcutaneous primary tumor, or metastatic tumor), Ki-67 expression, mitotic index (number of mitoses per 10 high-power fields [HPF]), and positive reaction to immunohistochemical stains (including vimentin, desmin, HHF-35 [muscle-specific actin], smooth muscle actin [SMA], S100, and CD34).
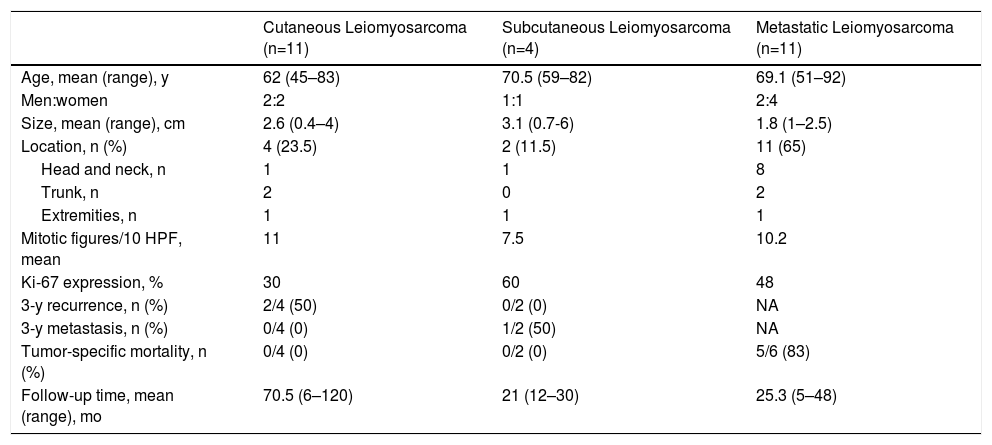
ResultsClinical and histopathologic data and clinical course recorded for the included cases are shown in Table 2.
Clinical and Histopathologic Features in 17 Cutaneous, Subcutaneous and Metastatic Leiomyosarcomas.
| Cutaneous Leiomyosarcoma (n=11) | Subcutaneous Leiomyosarcoma (n=4) | Metastatic Leiomyosarcoma (n=11) | |
|---|---|---|---|
| Age, mean (range), y | 62 (45–83) | 70.5 (59–82) | 69.1 (51–92) |
| Men:women | 2:2 | 1:1 | 2:4 |
| Size, mean (range), cm | 2.6 (0.4–4) | 3.1 (0.7-6) | 1.8 (1–2.5) |
| Location, n (%) | 4 (23.5) | 2 (11.5) | 11 (65) |
| Head and neck, n | 1 | 1 | 8 |
| Trunk, n | 2 | 0 | 2 |
| Extremities, n | 1 | 1 | 1 |
| Mitotic figures/10 HPF, mean | 11 | 7.5 | 10.2 |
| Ki-67 expression, % | 30 | 60 | 48 |
| 3-y recurrence, n (%) | 2/4 (50) | 0/2 (0) | NA |
| 3-y metastasis, n (%) | 0/4 (0) | 1/2 (50) | NA |
| Tumor-specific mortality, n (%) | 0/4 (0) | 0/2 (0) | 5/6 (83) |
| Follow-up time, mean (range), mo | 70.5 (6–120) | 21 (12–30) | 25.3 (5–48) |
Abbreviation: HPF, high-power field; NA, not applicable.
A total of 17 leiomyosarcomas in 12 patients (5 men, 41%; 7 women, 59%) were found in the registry. All patients were over 50 years of age at the time of diagnosis, and 45% were over the age of 70 years. The mean follow-up period after diagnosis of the cutaneous neoplasm (whether primary or metastatic) was 39.6 months.
There were 4 cutaneous leiomyosarcomas (23%) in 4 patients, 2 subcutaneous leiomyosarcomas (11.5%) in 2 patients, and 11 metastatic leiomyosarcomas (65%) in the remaining 6 patients. The metastatic tumors derived from primary tumors in the subfascial plane; in smooth muscle of the uterus or bladder; or in the intra-abdominal or retroperitoneal spaces. Metastatic tumor cells were found at an average of 1.8 sites per patient during follow-up.
The most frequent location at the time of diagnosis was the scalp in both the series as a whole (7/17, 41%) and in the subgroup of metastatic leiomyosarcomas. The lower limbs (3/17, 17%) and the trunk (3/17, 17%) were the next most common sites. The mean sizes of tumors on diagnosis in all 3 categories were as follows: cutaneous leiomyosarcomas, 2.6cm; subcutaneous leiomyosarcomas, 3.1cm; and metastatic leiomyosarcomas, 1.8cm.
Histology found a mean of 11 mitotic figures per 10 HPF and Ki-67 protein expression of 30% in cutaneous tumors, 7.5 mitotic figures per 10 HPF and Ki-67 expression of 60% in subcutaneous forms, and 10.2 mitotic figures per 10 HPF and Ki-67 expression of 48% in metastatic forms. Immunohistochemical staining was positive for vimentin (>25% of cases), desmin (>50%), HHF-35 (>75%), and SMA (>75%). S100 staining was focally positive in 1 cutaneous leiomyosarcoma; CD34 staining was also positive in 1 cutaneous tumor.
All the leiomyosarcomas in this series were excised surgically on diagnosis. Two of the cutaneous tumors did not recur after excision with margins greater than 1cm. However, the remaining cutaneous tumors did recur within 2 to 3 years after excision. The recurrences were excised, followed by radiation, and no further recurrences were registered during follow-up. One patient with a subcutaneous tumor remained free of disease. The second patient with this form developed multiple node, bone and muscle metastases and at the time of writing was receiving third-line chemotherapy (trabectedin).
The first focus of metastatic leiomyosarcoma cells in the third subgroup occurred 2 to 18 months after diagnosis of the primary tumor. In 2 cases metastasis to skin preceded metastasis to other sites, whereas in 3 cases it occurred afterwards. The generalized metastasis that developed during the course of disease in the 6 patients with metastatic skin leiomyosarcoma usually involved the lung. The next most commonly involved organ was the liver (3 patients). Five of these 6 patients died within an average of 25 months after diagnosis of their skin metastasis, in spite of chemo- and radiotherapy.
DiscussionCutaneous soft-tissue sarcomas—and leiomyosarcomas among them—are rare tumors. Because of the low incidence of these tumors, it is difficult to develop protocols for their diagnosis and management.
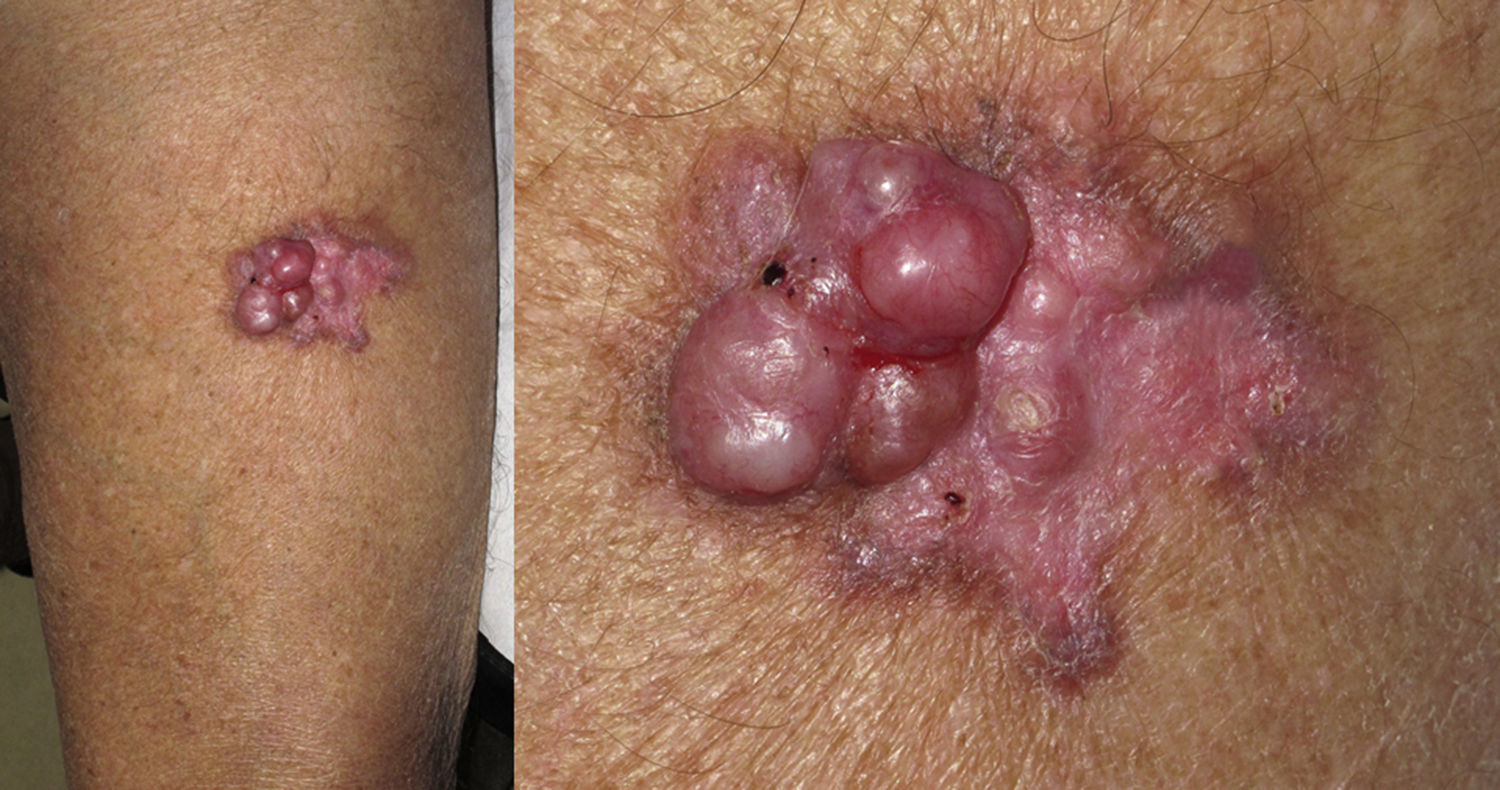
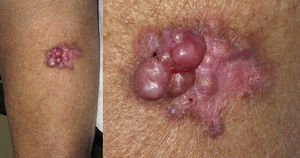
Cutaneous leiomyosarcomas usually present as nodules or masses that are firm to the touch (Fig. 1) in adults over the age of 50 years. Patients often consult the physician for pain, itching, or paresthesia in the area around the lesion (Fig. 1).
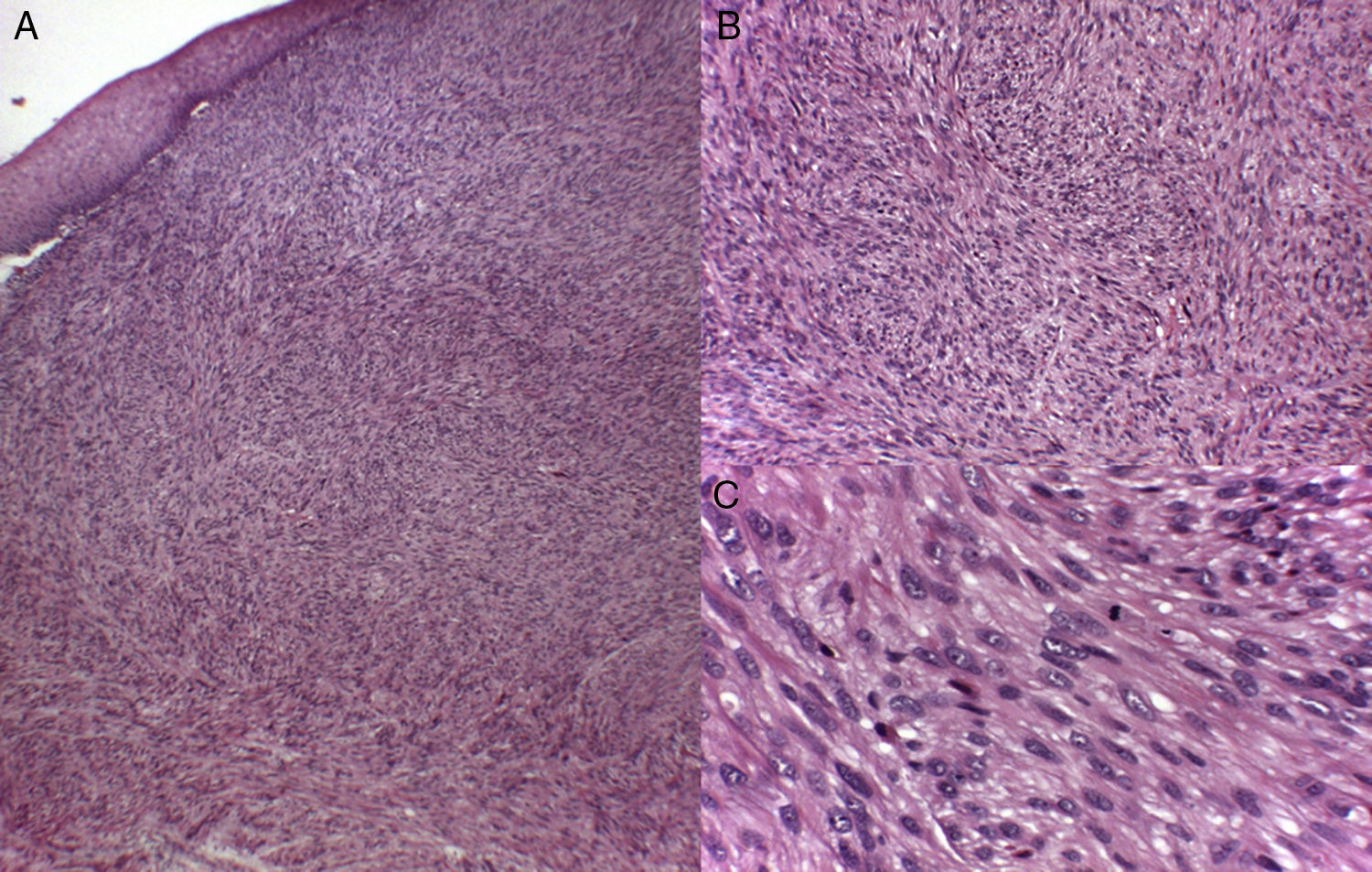
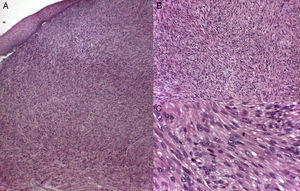
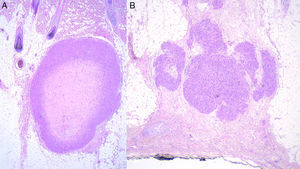
Histology recognizes 2 architectural patterns. One is a nodular pattern characterized by cellularity, cell atypia, and mitotic figures. The other is a diffuse pattern with lower cellularity and a lower mitotic index. Because cell atypia is not always evident, a histologic diagnosis should be supported by the overall architecture of the lesion (infiltrative pattern and high cellularity). It is particularly important to obtain adequate samples for histopathology, and incisional biopsies that extend below the dermis are preferred. Immunohistochemistry is an essential tool for distinguishing these neoplasms from tumors with fusiform cells and for establishing lineage11 (Fig. 2).
To date most of the literature on cutaneous leiomyosarcoma in the skin consists of descriptions of single cases or small series. Prognostic factors are therefore hard to discern, as this neoplasm varies in its clinical behavior.
Winchester et al2 analyzed prognostic indicators in one of the largest series of cutaneous and subcutaneous leiomyosarcomas published. They described 71 cases occurring over a period of 50 years. The most common location of both types of skin leiomyosarcoma was the extensor surfaces of the lower limbs. The next most common was the trunk. They found significant differences in the 5-year recurrence rate between cutaneous (18%) and subcutaneous (28%) forms. The median follow-up after diagnosis was 4 years. Distant metastasis was observed for 12% percent of the cutaneous and 51% of the subcutaneous tumors. The median latency from initial diagnosis to metastasis was 3 years. They also reported specific mortality for cutaneous and subcutaneous tumors (6% and 40%, respectively). Metastasis in this retrospective cohort occurred in 10.4% of the cutaneous leiomyosarcomas, obliging the authors to question the appropriateness of the term “atypical intradermal smooth muscle neoplasms” proposed by Kraft et al.4 Another group, Jensen et al,12 also analyzed prognostic factors (among them tumor size, depth, and histologic grade) in a series of 41 cutaneous and subcutaneous leiomyosarcomas. Tumor size greater than 5cm at diagnosis was the only independent variable to survive as a prognostic factor on multivariate analysis.
The features we report in our series are generally consistent with those earlier reports. However, the most frequent location in our patients was the head, attributable to our inclusion of metastatic leiomyosarcomas. In addition, we saw differences in the mitotic index, and the 2 recurring cutaneous leiomyosarcomas in our series had disease-free intervals of 2 and 3 years. One of our 2 patients with subcutaneous tumors remained free of disease after a year of follow-up in spite of an initial tumor size of 5cm. The higher mean mitotic index in cutaneous versus subcutaneous and metastatic tumors in our series was an interesting finding, although it is true that we have only been able to analyze a small number of cases.
Leiomyosarcoma that metastasizes to the skin has been reported in the literature13-16 and an analysis of 21 such cases was recently published by Winchester et al.5 Consistent with our observations, they found that the head was the most frequent location, probably because the high degree of vascularization in the scalp facilitates spread via the bloodstream. They also reported a mean age at diagnosis of the first metastatic tumor of 68 years, a median disease-free interval between tumor diagnosis and metastasis of 39 months, and a mean survival after the diagnosis of metastatic disease of 16 months. Our observations were similar. The mean age of our patients was 69.1 years, the median disease-free interval was 36 months after excision, and the mean survival after metastasis was 25 months—suggesting that metastasis to the skin from noncutaneous leiomyosarcomas indicates a poor prognosis.
Surgical treatment is the gold standard for localized tumors.17–19 However, important structural limitations make it difficult to determine the optimal size of safety margins. Aggressive surgery, with margins ranging from 3 to 5cm are traditionally recommended, but time has shown that a more conservative approach taking margins of 1cm can give similar results without increasing the rate of local recurrence. Mohs micrographic surgery seems to have gained special importance in this setting. During follow-up in a series of 41 leiomyosarcomas treated with Mohs surgery, only 2 cases of recurrence and no cases of metastasis were observed.18–21 Nevertheless, prospective studies in larger series and longer periods of follow-up are needed to confirm those findings. The role of radiotherapy in leiomyosarcoma has not been clearly defined to date, but this option can prove important when we treat deep tumors with a poor prognosis or when the margins surrounding excised tumors are not disease-free.17,22 Neoadjuvant, adjuvant, or palliative therapy is currently indicated for nonresectable tumors or disseminated disease, depending on tumor stage.17,23,24 All lesions in our series could be removed with surgical margins between 3 and 5cm except in cases of confirmed metastasis, in which we removed tumors with 1-cm margins and prescribed chemotherapy.
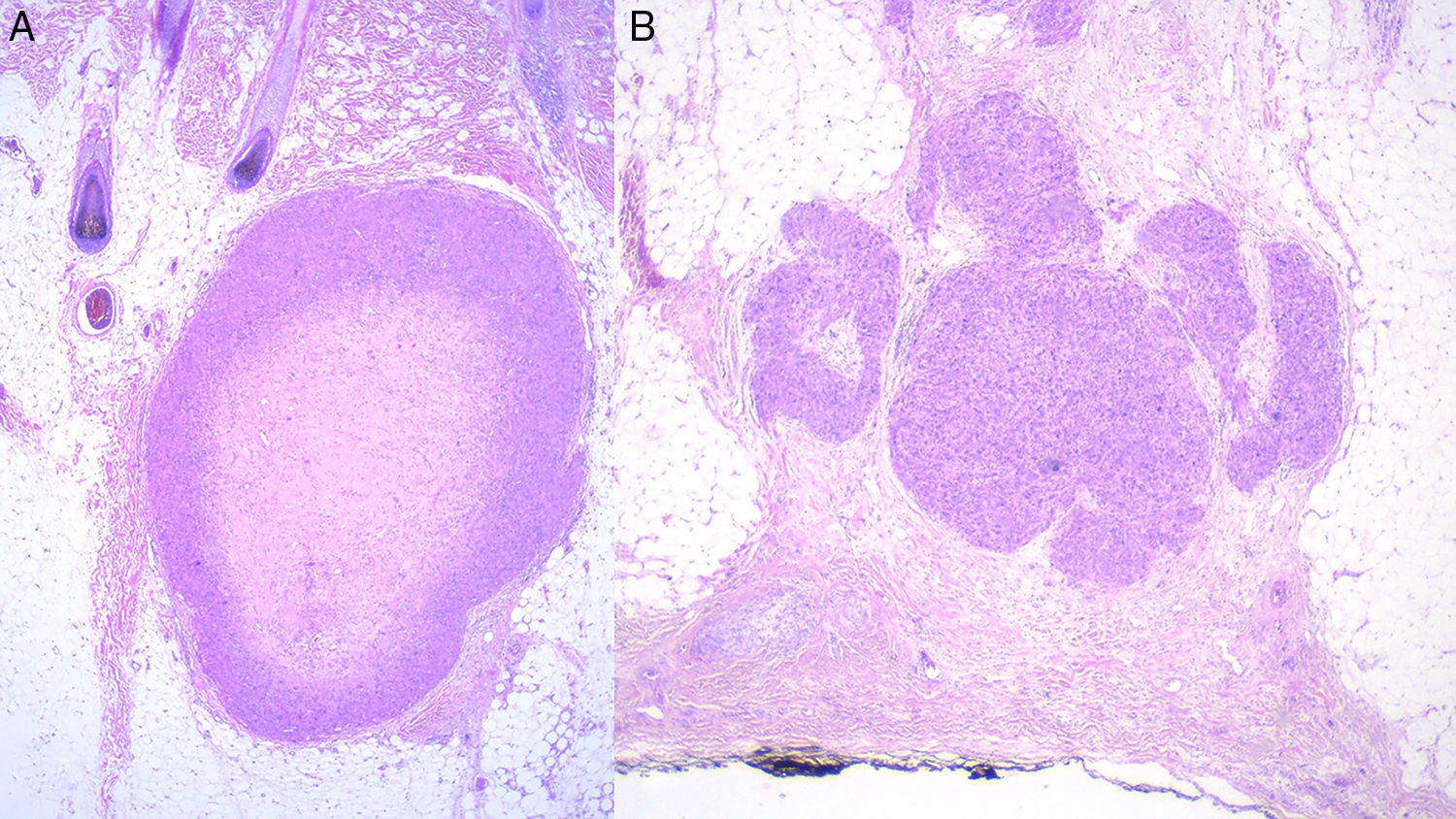
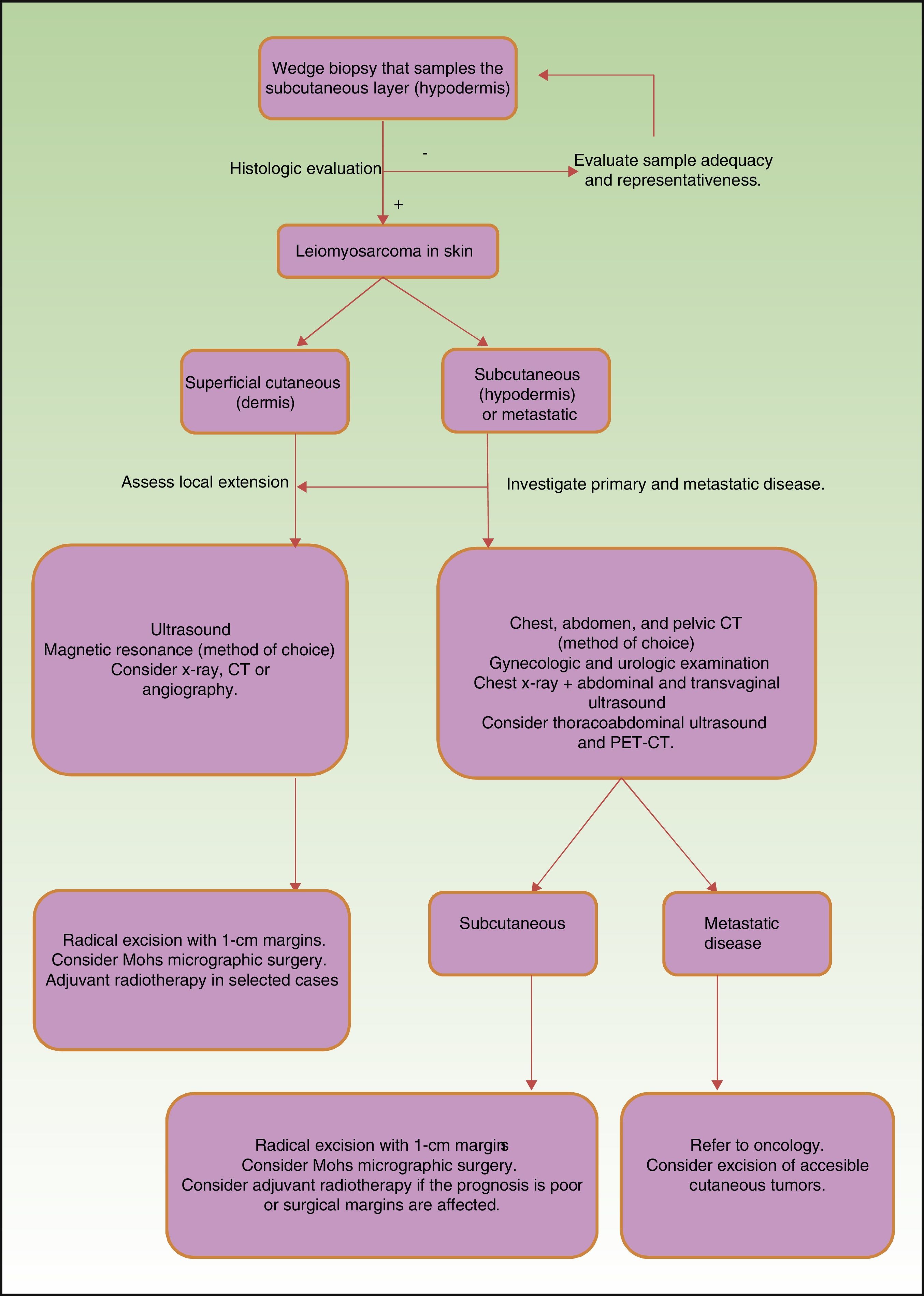
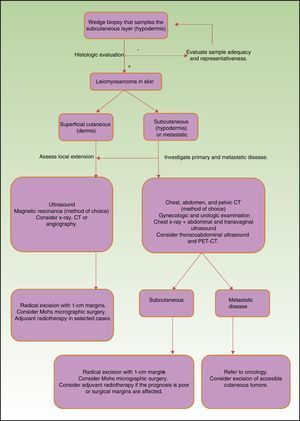
Prognosis depends mainly on subtype: true cutaneous forms, cutaneous forms that penetrate subcutaneous tissue, subcutaneous forms, and metastatic forms. For this reason cutaneous leiomyosarcomas have been excluded from the World Health Organization's classification system for soft-tissue sarcomas.9 However, these distinctions are not present in the main diagnostic algorithms used in clinical practice (the 2016 version of the National Comprehensive Cancer Network Guidelines17), which refer to leiomyosarcomas without reference to subtypes, grouping them together with other high-grade, aggressive sarcomas. Therefore, given the lack of specific recommendations, the clinician must make individualized decisions when ordering images to evaluate tumor extension. Furthermore, even though metastatic skin involvement arising from leiomyosarcomas is even less frequent than primary leiomyosarcoma, the clinical and histopathologic differential diagnosis between these forms can be complicated (Fig. 3). The possibility of an unidentified primary visceral tumor should therefore be investigated in all cases of leiomyosarcoma confined to subcutaneous tissue. Fig. 4 shows a possible diagnostic algorithm for individualized diagnosis based on clinical and histopathologic features. The algorithm can provide guidance on choosing complementary tests and images to order early in the diagnostic process.
In conclusion, leiomyosarcoma of the skin is a rare malignancy with clearly defined prognostic differences according to histologic subtype and whether the tumor is primary or metastatic. Even though this series analyzes few cases and data was collected retrospectively, we have been able to construct an algorithm to guide diagnosis and management that we believe will be useful to dermatologists who must treat this rare but potentially aggressive tumor.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Lomba E, Molina-López I, Parra-Blanco V, Suárez-Fernández R, Pulido-Pérez A. Leiomiosarcoma cutáneo: características clínicas, histopatológicas y correlación pronóstica en 12 pacientes. Actas Dermosifiliogr. 2018;109:140–147.