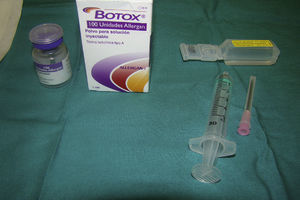
An approved indication for botulinum toxin (BT) is for the treatment of severe and persistent primary hyperhidrosis of the axilla that interferes with the activities of daily living and that is resistant to topical treatment. This is currently the only approved indication for botulinum toxin type A (BTA), marketed as Botox and presented in the form of vials containing 100units.1,2
Prior to indicating this procedure, the medical history must be taken, a physical examination performed, and specific additional tests done as required to exclude possible causes of secondary hyperhidrosis and avoid the symptomatic treatment of hyperhidrosis without the diagnosis and/or treatment of an underlying disease.2,3
Clinical improvement usually develops within a week after the injection.2–4 The injection of BT can be repeated when the clinical effect of the previous injection declines and the specialist considers it necessary; however, it is not advisable to repeat injections at intervals of less than 16 weeks.1
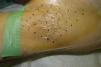
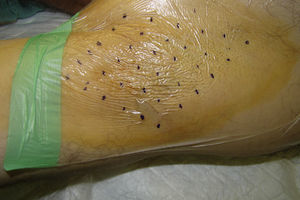
Description of the TechniquePrior to injection, the area of axillary hyperhidrosis to be treated can be identified using the starch-iodine technique (the Minor test). The quadrants to be treated are defined and antiseptic is applied. The quadrants can be marked on a transparent dressing rather than on the skin in order to avoid the risk that the marks become blurred by the sweat.5 The discomfort associated with the procedure can be minimized by the subcutaneous injection of local anesthetic, or topical anesthesia can be applied prior to starting the procedure.
The vial of 100units of BTA is reconstituted by dissolution in 0.9% saline solution (Fig. 1); though the volume of solution for reconstitution of the vial can be varied, it is usually done with 5mL, which will give us a concentration of 2units in every 0.1mL of the resulting solution.
As a rule, 50U are administered into each axilla,1 injected intradermally using sterile 30gauge needles, making the injections as equally spaced as possible over the area of hyperhidrosis, separated by 1 to 2cm (depending on the size of the area to be treated). Typically, 25 injections are made per axilla (Fig. 2), injecting 0.1mL (2units) at each site.3
Precautions and ContraindicationsThere is no approved indication for the use of BTA in the treatment of hyperhidrosis of the axilla in patients under 18 years of age or in pregnant or breastfeeding women as there is insufficient data concerning safety and efficacy.1
The use of BTA in patients with neuromuscular disorders must be performed with extreme caution and under strict supervision due to the risk of onset of excessive muscle weakness.1,3
The procedure is contraindicated in patients with known hypersensitivity to BTA or to any of the excipients of the preparation and in patients with infection in the area of the proposed sites of injection.1,3
In theory, the effect of BT can be potentiated by the aminoglycoside antibiotics and spectinomycin, as well as by other drugs that interfere with neuromuscular transmission, such as the neuromuscular blocking agents.1,3
Variations in the clinical effect after repeated use of BTA may be due to the use of different procedures for reconstitution of the vial, different intervals between injection, and slight variations in the potency of the toxin due to the biological process used for its synthesis. The formation of neutralizing antibodies to BTA can reduce the effectiveness of treatment; injection of the minimum effective dose at the maximum possible interval between injections is therefore recommended to minimize the risk of formation of these antibodies.1
ComplicationsComplications of this technique are uncommon (11% according to the summary of product characteristics1) and the large majority are minor and transitory.2–4 As with any injection, the technique can give rise to infection, pain, inflammation, paresthesia, hypoesthesia, swelling, edema, erythema, and localized hemorrhage or hematomas (often related more to the previous injection of local anesthetic).1
The pain associated with insertion of the needles and/or anxiety can give rise to vasovagal reactions. In very rare cases (0.7%), transitory muscle weakness can develop in the upper limb. In addition, about 4.5% of patients develop excess sweating in untreated areas (including areas without previous hyperhidrosis), and this can persist for up to a month.1
Severe or immediate hypersensitivity reactions, including anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea have been reported rarely.1,3
ConclusionsThe injection of botulinum toxin for the treatment of axillary hyperhidrosis is a simple procedure that is relatively quick to perform and has a low risk of complications.2,4 The technique produces a high level of satisfaction in a large majority of patients.2–4
The main limitations to the technique are the transitory nature of its effect and the cost of the vial of botulinum toxin.2,4
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: del Boz J, Padilla-España L, Segura-Palacios JM. Técnica de infiltración de toxina botulínica en hiperhidrosis axilar. Actas Dermosifiliogr. 2014;105:517–518.