The benign cutaneous plexiform hybrid tumor of perineurioma and cellular neurothekeoma (BCPHTPCN) is an entity described by Requena et al.1 in 2013. It typically presents clinically as a small solitary papule in the perioral region, although other authors have described a lesion on the nose2 and another on the left ankle.3 Histology reveals mixed pathological features of perineurioma and of cellular neurothekeoma with a plexiform architecture.1,3 Immunohistochemistry, when performed, has been positive for S100A6, microphthalmia-associated transcription factor (MiTF), lysosomal membrane-associated glycoprotein 3 (NKI/C3), protein gene product 9.5 (PGP9.5), epithelial membrane antigen (EMA), and neuron-specific enolase (NSE). Expression of CD34, claudin-1, and glucose transporter 1 (GLUT1) has been more variable, weaker, and focal. In addition to the 9 patients in the original study, a further 2 cases have been published. We present a new case of this recently identified lesion. The tumor in our case was positive for CD68 and CD163 in a large number of cells situated between the nests of neoplastic cells, a finding that was only described in a few stromal histiocytes in 2 of the cases in the original series.1
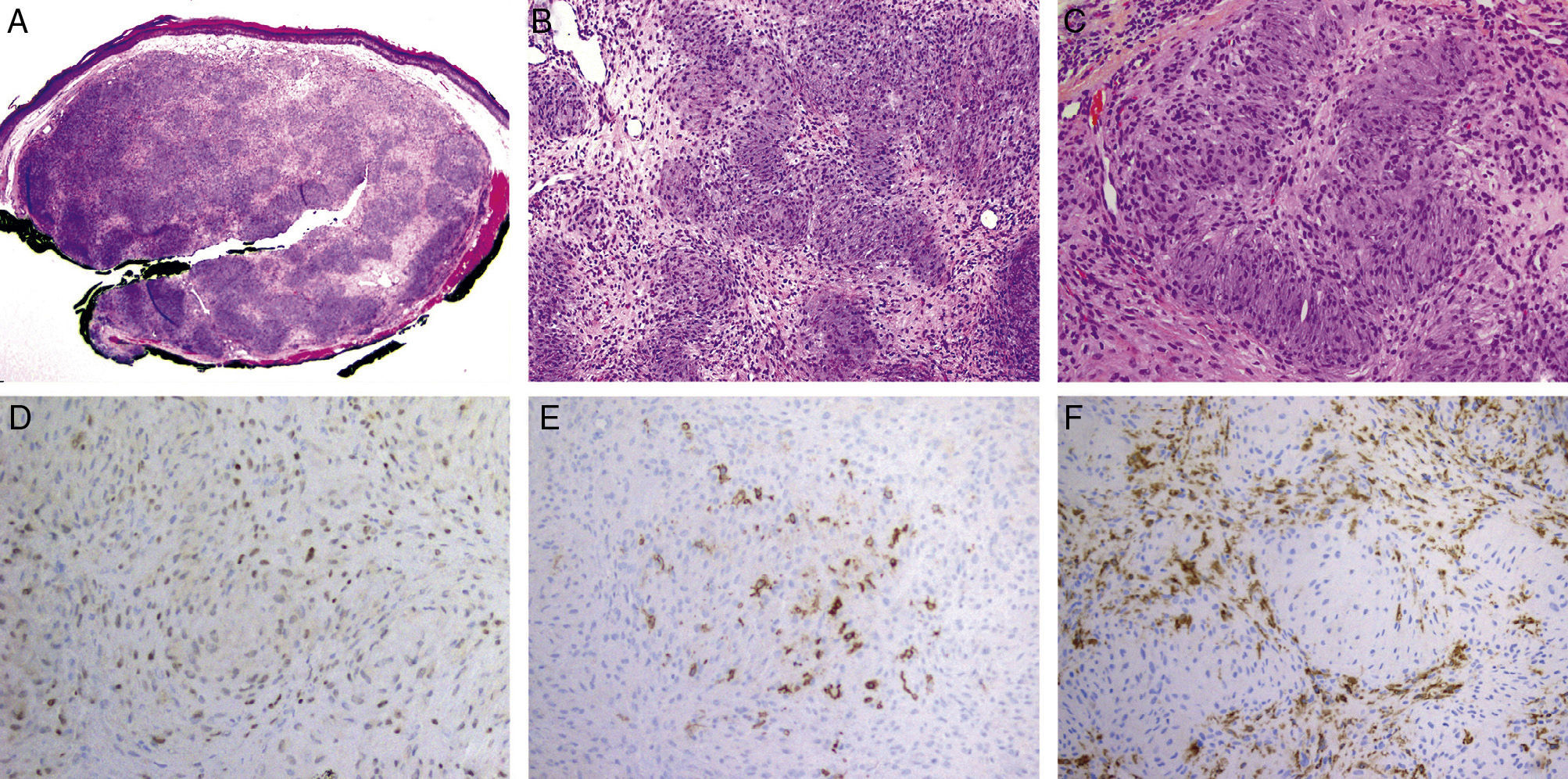
The patient was a man aged 58 years who presented a well-defined, hard, round, elevated lesion that had been growing slowly on his lower lip for 2 years and measured 5mm in diameter at the time of first consultation. The surface of the lesion was erythematous and shiny. The patient was otherwise asymptomatic. Curettage was performed. Histology showed a circumscribed, nonencapsulated nodule in the superficial dermis (Fig. 1A). The nodule had a plexiform architecture (Fig. 1B) and did not make contact with the epidermis. Some cells had an epithelioid appearance with abundant eosinophilic cytoplasm, blurred cell borders, vesicular nuclei, and inconspicuous nucleoli. Other cells were delicate and spindle-shaped, with hyperchromatic nuclei. No pleomorphism, mitoses, or necrosis were observed. The stroma was myxoid with areas of collagen appearance (Fig. 1C). The epidermis showed mild hyperpigmentation of the basal layer, hyperkeratosis, and pagetoid dyskeratosis.
A, In the superficial dermis, a circumscribed nonencapsulated nodule is observed that does not make contact with the epidermis. Hematoxylin and eosin (H&E), original magnification×2. B, Plexiform architectural pattern. The neoplastic cells form round and irregular nests in a predominantly myxoid stroma. No neural or vascular structures are visible within these nests. H&E, original magnification×10.C, The majority of the neoplastic cells have ovoid nuclei with inconspicuous nucleoli and eosinophilic cytoplasm with poorly defined borders. These cells are intimately associated with distinct spindle-shaped cells with delicate, elongated, hyperchromatic nuclei. D, Diffuse positivity for MiTF in the nuclei of the neoplastic cells. Original magnification×10. E, Focal positivity for claudin-1 in the neoplastic cells. Original magnification×10. F, Positivity for CD163 in numerous interstitial cells between the nests. Original magnification×10.
Immunohistochemistry was diffusely positive for vimentin and MiTF (Fig. 1D) and focally positive for claudin-1 (Fig. 1E). The tumor cells were negative for S100, Melan-A, NSE, EMA, GLUT1, actin, and CD34. Numerous CD68+ and CD163+ cells were observed between the nests (Fig. 1F). A diagnosis of BCPHTPCN was made.
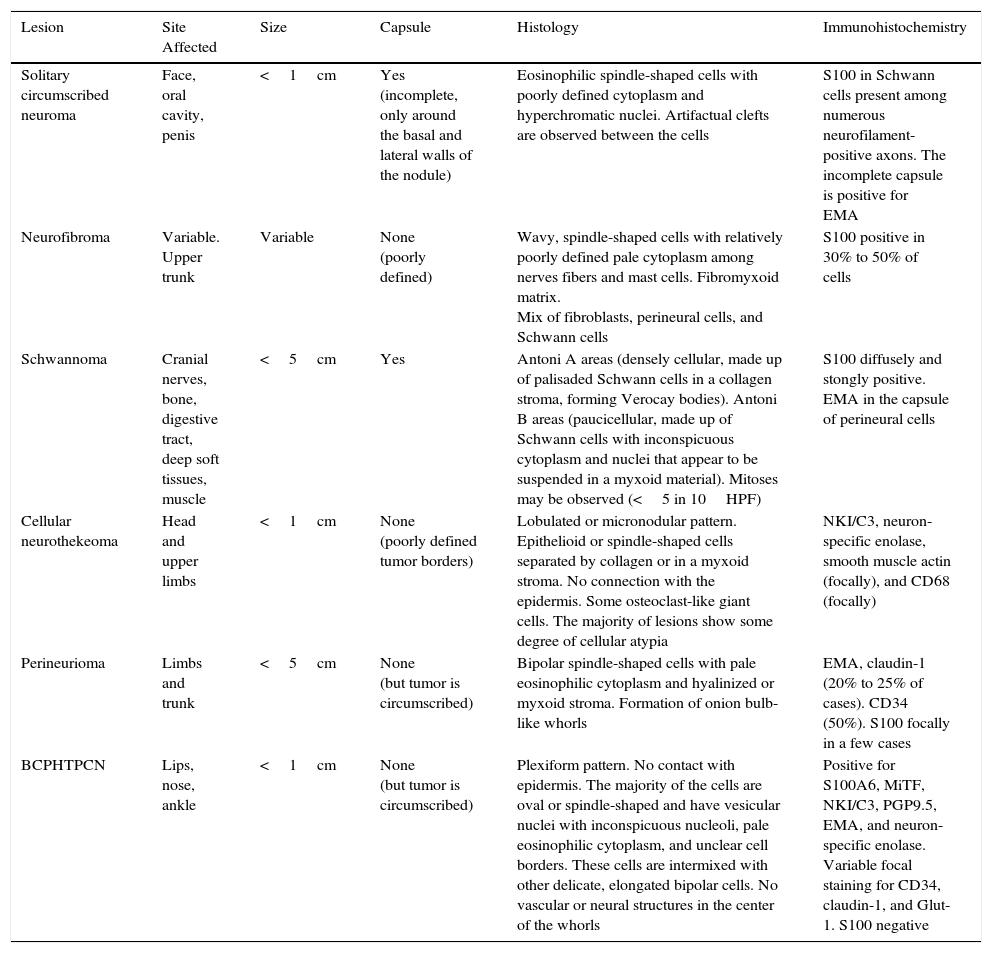
Perineurioma is a tumor formed of perineural cells arranged in whorls in a hyalinized or myxoid collagen stroma. Although immune expression of EMA is typical, these tumors are frequently positive for claudin-1, GLUT1, and CD34, and almost always negative for protein S100.3 Cellular neurothekeoma is a tumor of uncertain histogenesis, with a lobulated architectural pattern. It is formed of epithelioid and/or spindle-shaped cells in a predominantly myxoid stroma. It is usually positive for vimentin, NKI-C3, NSE, MiTF1, and PGP9.5, and sometimes for actin and CD68 (Table 1).4,5 In 2013, Requena et al.1 described BCPHTPCN as a tumor with mixed features of perineurioma and cellular neurothekeoma, with a plexiform architectural pattern. The original series consisted of 9 cases in which the perioral region was affected, as found in our case. However, Yamada et al.2 subsequently reported a case affecting the nose, and Linos et al.3 described one on the left ankle. To date, all reported patients have been adults aged between 30 and 76 years, with no gender difference. Clinically the tumor presents as a solitary papule or nodule covered by skin of normal appearance1–3; the histopathology findings have also been similar in all the lesions, with the exception of the case presented by Linos et al.,3 in which the plexiform architecture was not observed.
Summary of the Main Differential Diagnoses.
| Lesion | Site Affected | Size | Capsule | Histology | Immunohistochemistry |
|---|---|---|---|---|---|
| Solitary circumscribed neuroma | Face, oral cavity, penis | <1cm | Yes (incomplete, only around the basal and lateral walls of the nodule) | Eosinophilic spindle-shaped cells with poorly defined cytoplasm and hyperchromatic nuclei. Artifactual clefts are observed between the cells | S100 in Schwann cells present among numerous neurofilament-positive axons. The incomplete capsule is positive for EMA |
| Neurofibroma | Variable. Upper trunk | Variable | None (poorly defined) | Wavy, spindle-shaped cells with relatively poorly defined pale cytoplasm among nerves fibers and mast cells. Fibromyxoid matrix. Mix of fibroblasts, perineural cells, and Schwann cells | S100 positive in 30% to 50% of cells |
| Schwannoma | Cranial nerves, bone, digestive tract, deep soft tissues, muscle | <5cm | Yes | Antoni A areas (densely cellular, made up of palisaded Schwann cells in a collagen stroma, forming Verocay bodies). Antoni B areas (paucicellular, made up of Schwann cells with inconspicuous cytoplasm and nuclei that appear to be suspended in a myxoid material). Mitoses may be observed (<5 in 10HPF) | S100 diffusely and stongly positive. EMA in the capsule of perineural cells |
| Cellular neurothekeoma | Head and upper limbs | <1cm | None (poorly defined tumor borders) | Lobulated or micronodular pattern. Epithelioid or spindle-shaped cells separated by collagen or in a myxoid stroma. No connection with the epidermis. Some osteoclast-like giant cells. The majority of lesions show some degree of cellular atypia | NKI/C3, neuron-specific enolase, smooth muscle actin (focally), and CD68 (focally) |
| Perineurioma | Limbs and trunk | <5cm | None (but tumor is circumscribed) | Bipolar spindle-shaped cells with pale eosinophilic cytoplasm and hyalinized or myxoid stroma. Formation of onion bulb-like whorls | EMA, claudin-1 (20% to 25% of cases). CD34 (50%). S100 focally in a few cases |
| BCPHTPCN | Lips, nose, ankle | <1cm | None (but tumor is circumscribed) | Plexiform pattern. No contact with epidermis. The majority of the cells are oval or spindle-shaped and have vesicular nuclei with inconspicuous nucleoli, pale eosinophilic cytoplasm, and unclear cell borders. These cells are intermixed with other delicate, elongated bipolar cells. No vascular or neural structures in the center of the whorls | Positive for S100A6, MiTF, NKI/C3, PGP9.5, EMA, and neuron-specific enolase. Variable focal staining for CD34, claudin-1, and Glut-1. S100 negative |
Abbreviations: BCPHTPCN, benign cutaneous plexiform hybrid tumor of perineurioma and cellular neurothekeoma; EMA, epithelial membrane antigen; GLUT-1, glucose transporter 1; HPF, high-power fields; MiTF, microphthalmia-associated transcription factor; NKI/C3, lysosomal membrane-associated glycoprotein 3; S100, protein S100.
The tumor in our patient showed all the typical morphological characteristics and was diffusely positive for MiTF1 (specific to neurothekeoma) and focally positive for claudin-1 (commonly observed in perineurioma). However, other markers frequently used for the diagnosis of these tumors—NSE, S100, EMA, GLUT1, actin, and CD34—were negative. Staining for CD68 and CD163 was positive in numerous cells distributed between the nests of the tumor. This finding was described in 2 of the cases presented by Requena et al.,1 but only affected a few stromal histiocytes. In all cases of BCPHTPCN published to date (including our case), protein S100, melanoma markers (HMB45, Melan-A, SOX10), and smooth muscle markers (actin, desmin) have been negative. MiTF1, S100A6, and PGP9.5 have been strongly positive in those cases in which they were studied. NKI/C3, EMA, NSE, CD34, claudin-1, GLUT1, and CD68 have shown more variable results in BCPHTPCN.
In conclusion, BCPHTPCN is a recently described tumor that shows a preference for the perioral region, and whose pathology findings are a hybrid between perineurioma and cellular neurothekeoma. Immunohistochemistry is variable, but important findings include positivity for S100A6, MiTF, NKI/C3, PGP9.5, EMA, and NSE. In our case, we draw particular attention to the presence of numerous CD68+ and CD163+ cells between the tumor nests. This peculiar trait has been reported previously in 2 patients, but as a less intense feature.1 Given the variability of the immunohistochemistry findings and, in general, the low levels of expression of typical neural markers in this neoplasm, we consider the clinical and morphological features to be key elements in reaching the diagnosis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors would like to thank the Dermatology Department and the technicians of the Pathology Department of Complejo Hospitalario de Navarra.
Please cite this article as: Areán C, Córdoba A, Requena L, Álvarez ML. Tumor benigno cutáneo plexiforme híbrido de perineuroma y neurotecoma celular. Actas Dermosifiliogr. 2016;107:607–610.