Psoriasis is a chronic disease with a prevalence of between 1% and 3%. Its clinical manifestations and associated comorbidities have a considerable impact on affected patients.
While the advent of biologic therapy has greatly improved the control of moderate to severe psoriasis,1 the treatment of this disease is still complicated. We have data on the patients who participated in clinical trials of biologic drugs, but these cases are often unlike those we encounter in clinical practice, as shown by a study of data from the Spanish BIOBADADERM registry.2 That study, which analyzed data from 1042 patients on systemic therapy (classic and biologic), reported that 30% of those patients would have been excluded from clinical trials. The patients who would not have been eligible for inclusion in a clinical trial had a higher risk of developing severe adverse reactions to systemic treatment.
Since we consider that it is important to report data on patients being treated in routine clinical practice, we designed a study to evaluate the efficacy, safety, and survival of treatment with ustekinumab in our hospital. We studied patients with moderate to severe psoriasis who started treatment with ustekinumab in our specialized psoriasis clinic between 1 January 2010 and 30 April 2017.
The characteristics of the patients included in the study are shown in Table 1. Patients weighing 100 kg or less started treatment with an initial dose of ustekinumab 45mg followed by a second dose at 4 weeks and then 45mg every 12 weeks for at least 12 months. At 1 year, treatment optimization was considered, taking into account the efficacy achieved. In 2 patients who weighed over 100kg, a dose of 90mg was administered initially followed by another at 4 weeks and 90mg every 12 weeks thereafter. Informed consent was obtained from all of the patients before start of treatment.
Patient Characteristics.
| Total number of patients n=69 | ||
| Women, 31.88% (21); Men, 69.57% (48) | ||
| Naive patients, 39% (28); Non-naive patients, 61% (41) | ||
| % patients = 1 prior biologic, 34.78%; > 1 prior biologic, 23.18% | ||
| Mean | Median | |
| Age on starting ustekinumab, y | 47.91 | 49 |
| Baseline PASI score | 13.34 | 12 |
| Final PASI score | 3.53 | 2 |
| Number of prior biologic therapies | 1.63 | 1 |
| Duration of follow-up, mo | 36.12 | 23.79 |
| Patients Still on Therapy and Reasons for Withdrawal | Number | % |
| Patients still on UST | 52 | 75.36 |
| Withdrawal due to adverse events | 2 | 2.90 |
| Withdrawal due to lack of efficacy | 9 | 13.04 |
| Withdrawal due to good response | 3 | 4.35 |
| Withdrawal because patient was lost to follow-up | 3 | 4.35 |
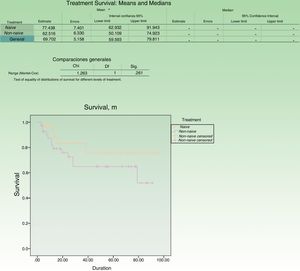
Treatment survival was calculated as the number of days a patient continued on ustekinumab during follow-up. The longest interval between two injections of the drug considered not to constitute loss to follow-up or treatment interruption was 180 days. We performed 2 analyses: one of the dataset as a whole and the other differentiating between patients who had previously received biologic therapy (naive) and those who had not (non-naive). The data was analyzed using the Kaplan-Meier method.
Treatment resulted in an improvement from the mean baseline Psoriasis Area and Severity Index (PASI) of 13.3 to a final mean PASI score of 3.5, which represents an 83% reduction in absolute PASI.
The treatment survival rate at the end of the 8.3 years of follow-up was 75.3%, and the rate was higher in the group of patients who had not previously been treated with a biologic agent than in the non-naive group (80% vs 60%) (Fig. 1).
Table 1 includes the patients who continued on ustekinumab and those in whom treatment was withdrawn. In this series, 6 patients reported adverse events, most of which were considered to be mild and did not lead to cessation of treatment. Treatment was withdrawn due to an adverse event in 2 patients: one who presented with infectious spondylodiscitis and another who developed severe palmoplantar pustulosis. A patient who developed psoriatic arthritis 3 months after starting ustekinumab continued on treatment, achieving a good response in terms of both skin lesions and joint involvement.
Tuberculin skin test conversion occurred in 12 patients, who were prescribed 6 to 9 months of treatment with isoniazid. No cases of active tuberculosis were recorded.
Several recent studies, including the PSOLAR3 and BABDIR4 studies, have collected data on the survival of biologic therapy in clinical practice. The findings of the Spanish ORBIT5 study—based on data from 427 patients collected over a 4-year period—highlight the efficacy and safety of long-term biologic therapy.
The treatment survival rate in this series was high (76.3% at 100 months). The 83% decline in absolute PASI score was similar to results reported by other authors in the literature. Puig et al.,6 in a 3-year study of 67 patients receiving ustekinumab, reported a PASI 75 response at 1 year in 86% of the patients. Also of interest is the study by Molina-Leyva et al.7 of 30 patients treated with ustekinumab, in which the PASI 75 response at 1 year was 81.5%.
In the present series, we should note that the baseline PASI was probably underestimated because none of the patients underwent a washout period before start of treatment with ustekinumab.
Withdrawal of treatment due to lack of efficacy (both primary and secondary treatment failure) was 13%. This low percentage may be due to the practice in our clinic of having a “watch and wait” period before deciding on loss of efficacy or withdrawal of treatment and to our patient's high degree of satisfaction with the efficacy achieved.
In the literature we reviewed, we found no cases of paradoxical psoriatic reactions associated with the use of IL-23 inhibitors. By contrast, their use as an alternative treatment for such reactions is reported.8 We did, however, find a report of a case in which paradoxical psoriasis worsened following treatment with ustekinumab.9
The limitations of this study include its retrospective design and the use of the treatment survival rate as a measure of treatment success because this endpoint does not provide any information on how patients were included or take into account cases in which treatment was discontinued because the lesions had cleared.
Conflicts of InterestIrene Salgüero has participated in clinical trials and has been invited to give papers by Janssen, Abbvie, and Novartis.
Please cite this article as: Salgüero Fernández I, Gil MH, Sanz MS, Gullón GR. Análisis de supervivencia, eficacia y seguridad en psoriasis moderada–grave tratada con ustekinumab. Estudio observacional de 69 pacientes en la práctica clínica habitual. Actas Dermosifiliogr. 2019;110:244–246.