The advent of immune targeted therapies for cancer has radically changed the treatment and prognosis of many cancers. These drugs are called targeted therapies because they target specific pathophysiological mechanisms of cancer. This paradigm shift in cancer treatment, however, has resulted in new adverse dermatologic effects involving both the skin and its appendages. In the case of hair, targeted drugs can cause immune alterations and changes in hair growth, color, and shape. Because most targeted therapies are new, there is no single document describing all these adverse effects. We performed an exhaustive review of the literature to characterize adverse hair effects associated with the use of targeted therapies.
Las nuevas terapias inmunológicas dirigidas contra el cáncer han supuesto un cambio radical en el tratamiento y el pronóstico de muchas neoplasias. Estos medicamentos se dirigen de manera mucho más específica contra los mecanismos fisiopatogénicos del cáncer, por lo que adquieren el sobrenombre de «terapias diana». Este cambio de paradigma ha supuesto la aparición de nuevos efectos adversos dermatológicos, que afectan tanto la piel como sus anejos. Los efectos adversos en el pelo pueden manifestarse en alteraciones de su ciclo, forma, color o inmunología. Debido a que son tratamientos nuevos en su mayoría y no existe un documento que englobe todos estos efectos adversos, hemos realizado una exhaustiva revisión bibliográfica para caracterizar de manera concreta cuáles son los efectos adversos tricológicos que pueden inducir cada uno de estos fármacos.
Since their introduction, targeted therapies have radically changed the treatment of cancer. These drugs target specific molecules implicated in tumor growth and proliferation (Table 1). They represent a change in approach for combating cancer, given that they act more specifically than traditional cytotoxic agents. The introduction of these agents has also led to a change in the management of these patients and the adverse effects resulting from cancer therapy. Although the new targeted therapies are generally less toxic for hair, they also have side effects that may impact hair, whether it is the follicular cycle, form, or color, or cause immune disorders leading to hair loss.
Main Molecular Targets for Targeted Cancer Therapy and the Corresponding Agents.
| Target | Main Agents |
|---|---|
| EGFR | Afatinib, cetuximab, erlotinib, gefitinib, necitumumab, osimertinib, panitumumab, vandetanib |
| HER-2 | Trastuzumab, lapatinib, neratinib |
| PD-1/PD-L1/CTLA-4 | Nivolumab, pembrolizumab/atezolizumab, avelumab, durvalumab/ipilimumab |
| BRAF | Dabrafenib, vemurafenib |
| MEK | Trametinib, cobimetinib, binimetinib, selumetinib |
| KIT/BCR-ABL | Axitinib, imatinib, regorafenib/bosutinib, dasatinib, nilotinib, ponatinib |
| VEGF/VEGFR | Bevacizumab, pazopanib, sorafenib, sunitinib/lenvatinib, ramucirumab |
| Hedgehog | Vismodegib, sonidegib |
| Proteasome | Bortezomib, carfilzomib, ixazomib |
| mTOR | Everolimus, temsirolimus, sirolimus |
| CD52 | Alemtuzumab |
| ALK | Alectinib, ceritinib, crizotinib |
| HDAC | Belinostat, panobinostat, romidepsin, vorinostat |
| RANKL | Denosumab |
| PARP | Olaparib, rucaparib, veliparib, talazoparib, iniparib |
| CD30 | Brentuximab |
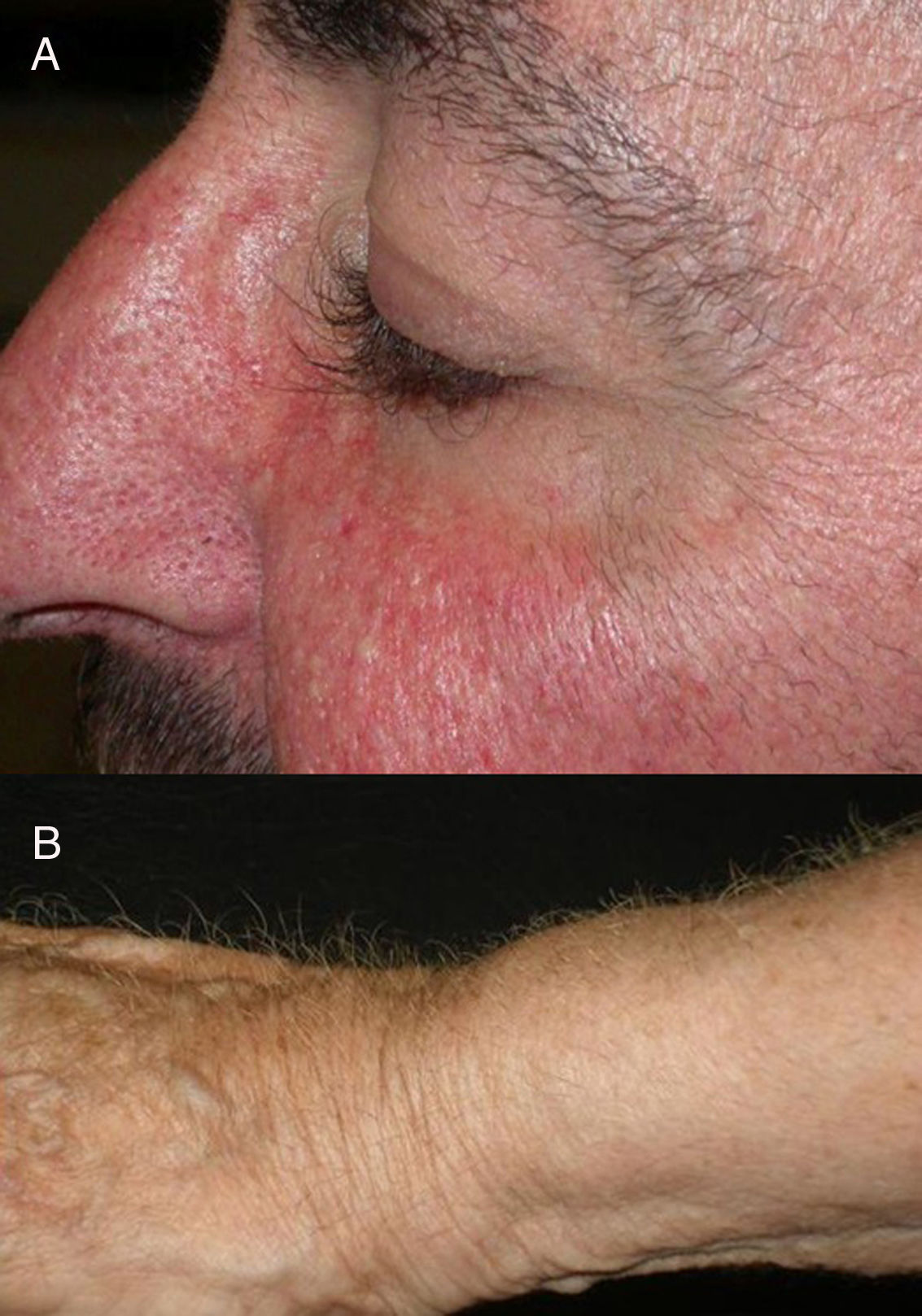
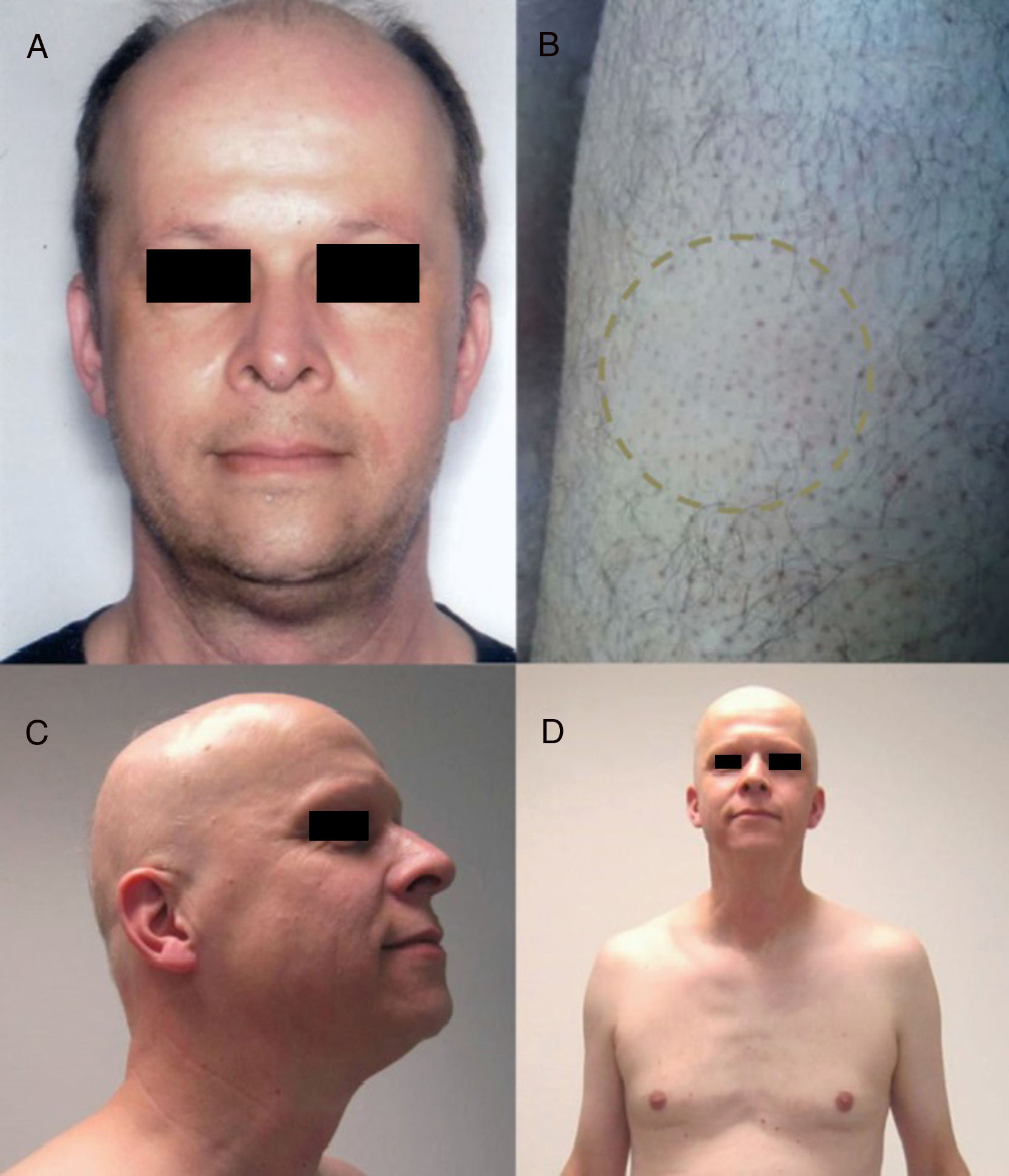
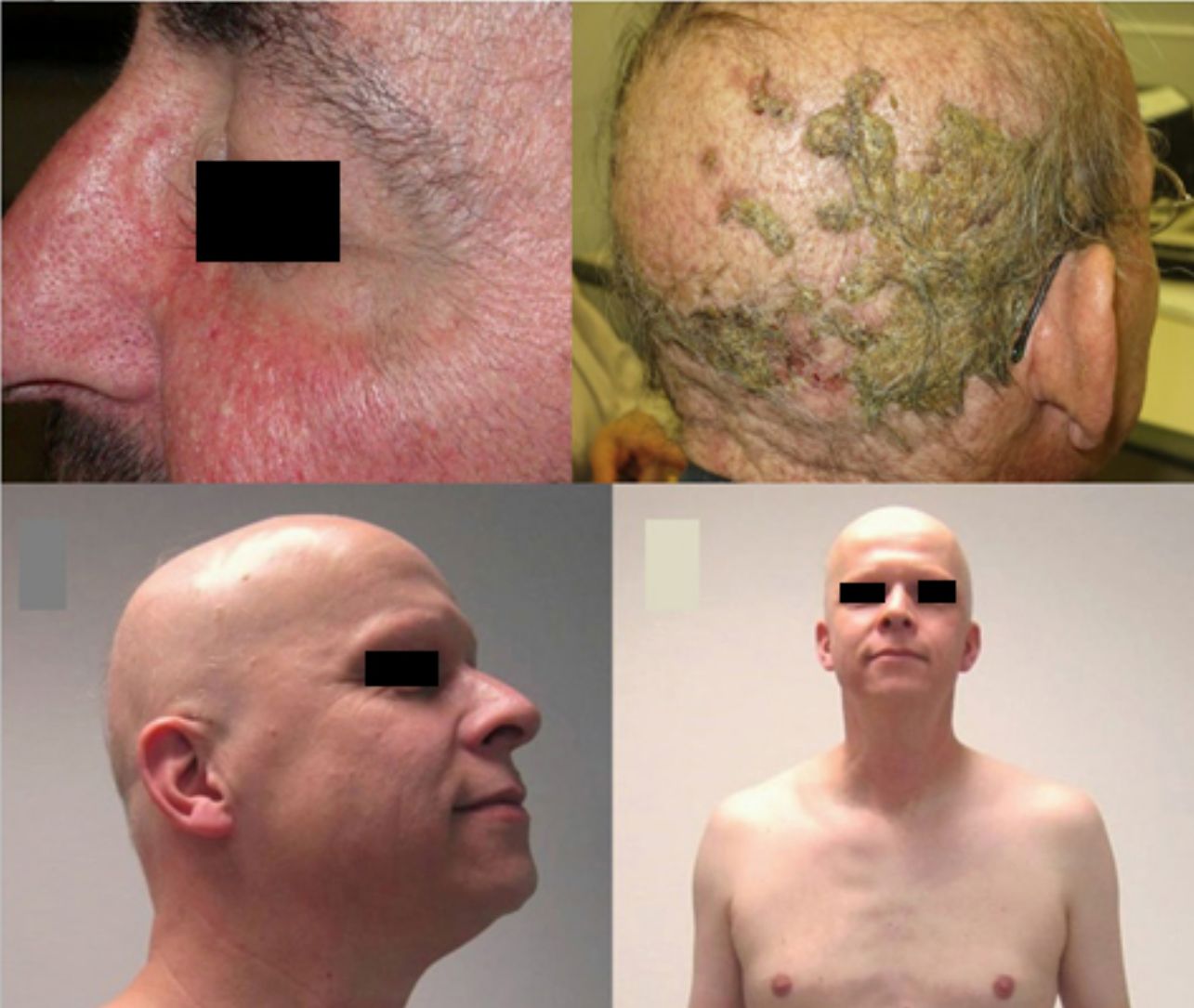
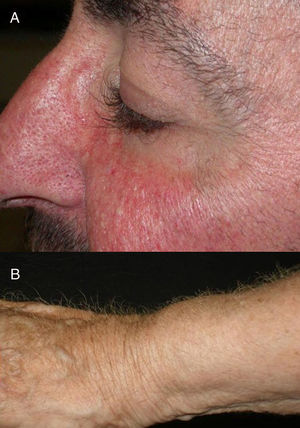
The role of epidermal growth factor receptors (EGFR) in tumor formation and development of skin and skin appendages is well known. Moreover, we know that this molecule plays an important role in the equilibrium of the follicular cycle.1 For this reason, the main adverse effects of these treatments are dermatological. These effects are essentially divided into those that affect the skin (follicular or acneiform eruption, xerosis, and pruritus), which usually have an early onset, and those that affect the appendages (paronychia and hair changes), which have a later onset.2–4 Hair changes occur in almost 80% of patients after 6 months of treatment. Several types of impact on hair have been reported: alopecia, hair growth, and changes in rate of growth, hair thickness, and even form, with hair taking on a curly or straight appearance.2 Rarer are changes in hair pigmentation. Two types have been reported: poliosis5 and repigmentation of grey hair.6 One of the most characteristic effects is, without doubt, persistent or accelerated hair growth, an effect known as trichomegaly.7 Hypertrichosis of the eye lashes is particularly striking and characteristic,5,7,8 and usually occurs between 2 and 5 months of treatment (Fig. 1). This abnormal growth, in addition to the cosmetic impact, leads to an aberrant form of eyelashes with the possibility, in some cases, of causing corneal trauma. For this reason, it is recommended to trim them carefully.4
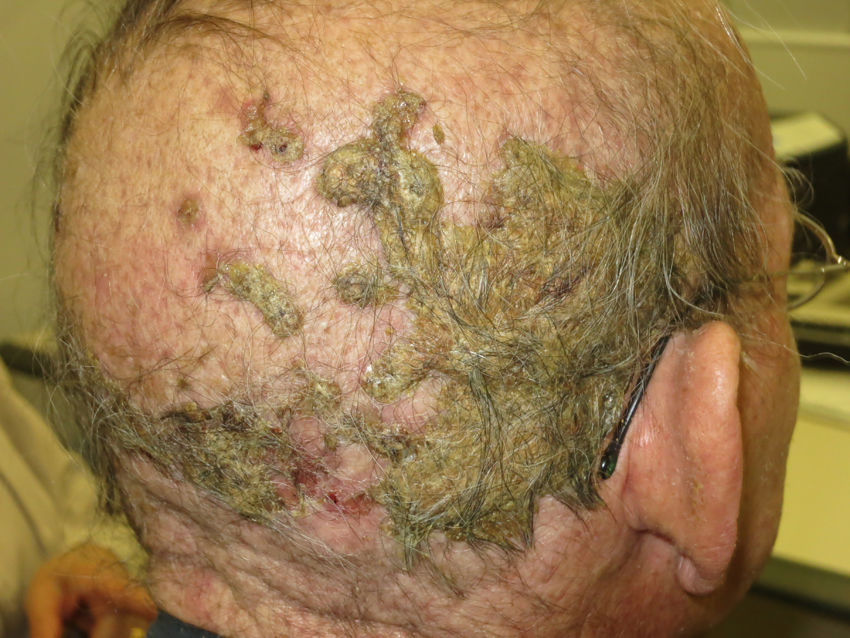
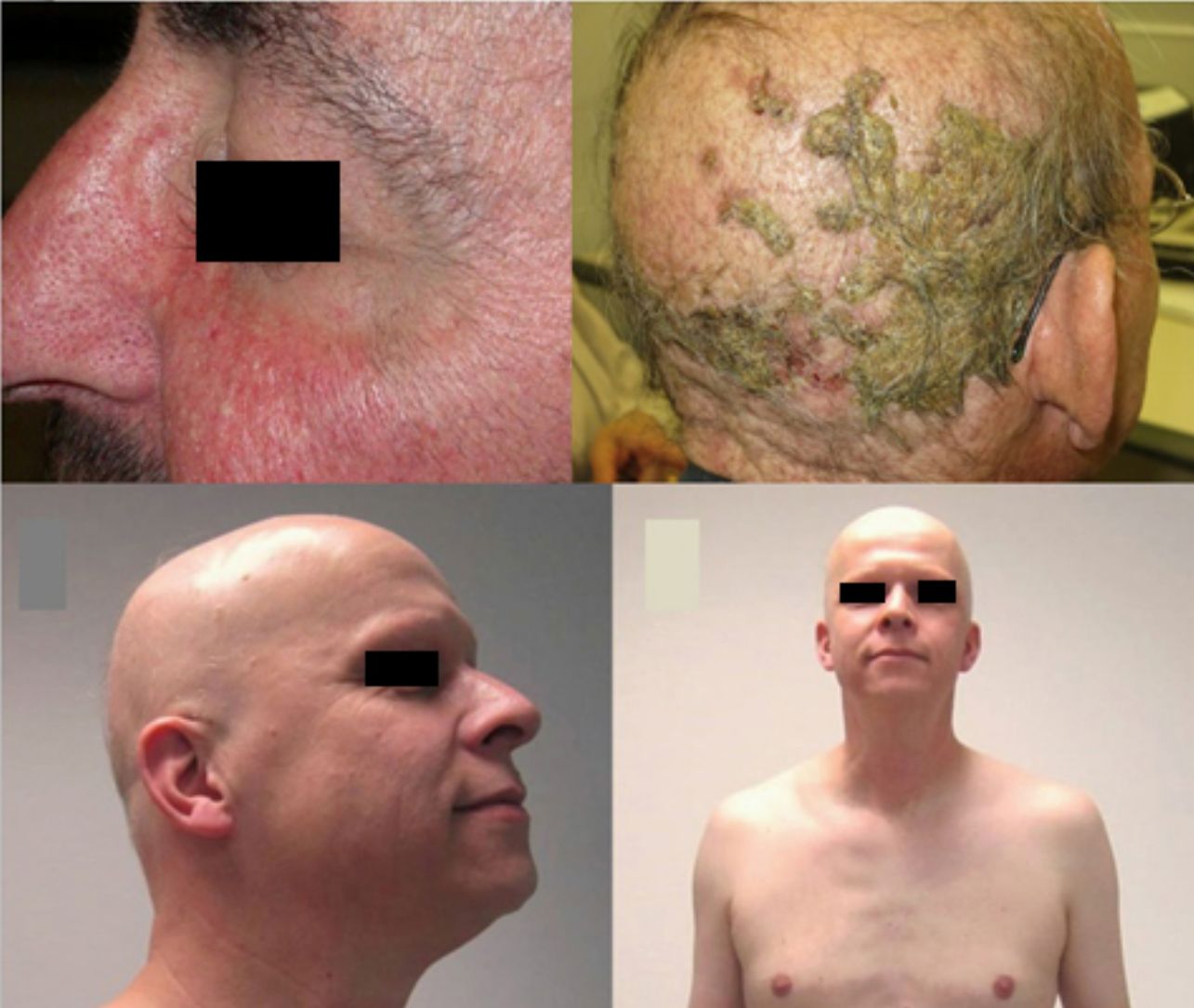
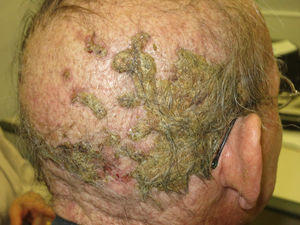
Alopecia related to these drugs occurs at different rates according to the drug: 11.9% with afatinib and 8.9% with cetuximab and erlotinib.9 In addition, less frequently, EGFR inhibitors can induce the onset of inflammatory alopecia. This may present as inflammatory nonscarring alopecia10 or as follicular pustules on the scalp, leading to scarring alopecia,4,11 which may correspond to scalp involvement in typical follicular reaction (Fig. 2). However, some authors are of the opinion that this is a form of erosive pustular dermatosis of the scalp because of the trichoscopic findings, characteristic of such processes.12 In any case, treatment is with topical corticosteroids, while addition of antibiotics with antistaphylococcal action may be useful as Staphylococcus aureus infection has been reported in some cases.11
HER-2 Inhibitors: Trastuzumab, Lapatinib, NeratinibWithin the pharmacological class of HER-2 inhibitors, trastuzumab is the most widely used drug as an adjuvant treatment for HER-2 positive breast cancer. Alopecia due to this drug does not appear to be a common adverse reaction.13 Lapatinib, in contrast, is an inhibitor of both HER-2 and EGFR, and so can show effects characteristic of this latter group of inhibitors.14 Clinical trials conducted with neratinib to date have not reported any effects on hair.15
Moreover, anecdotally, there have been 2 cases of tufted-hair folliculitis induced by HER-2 inhibitors, one with trastuzumab16 and the other with lapatinib.17
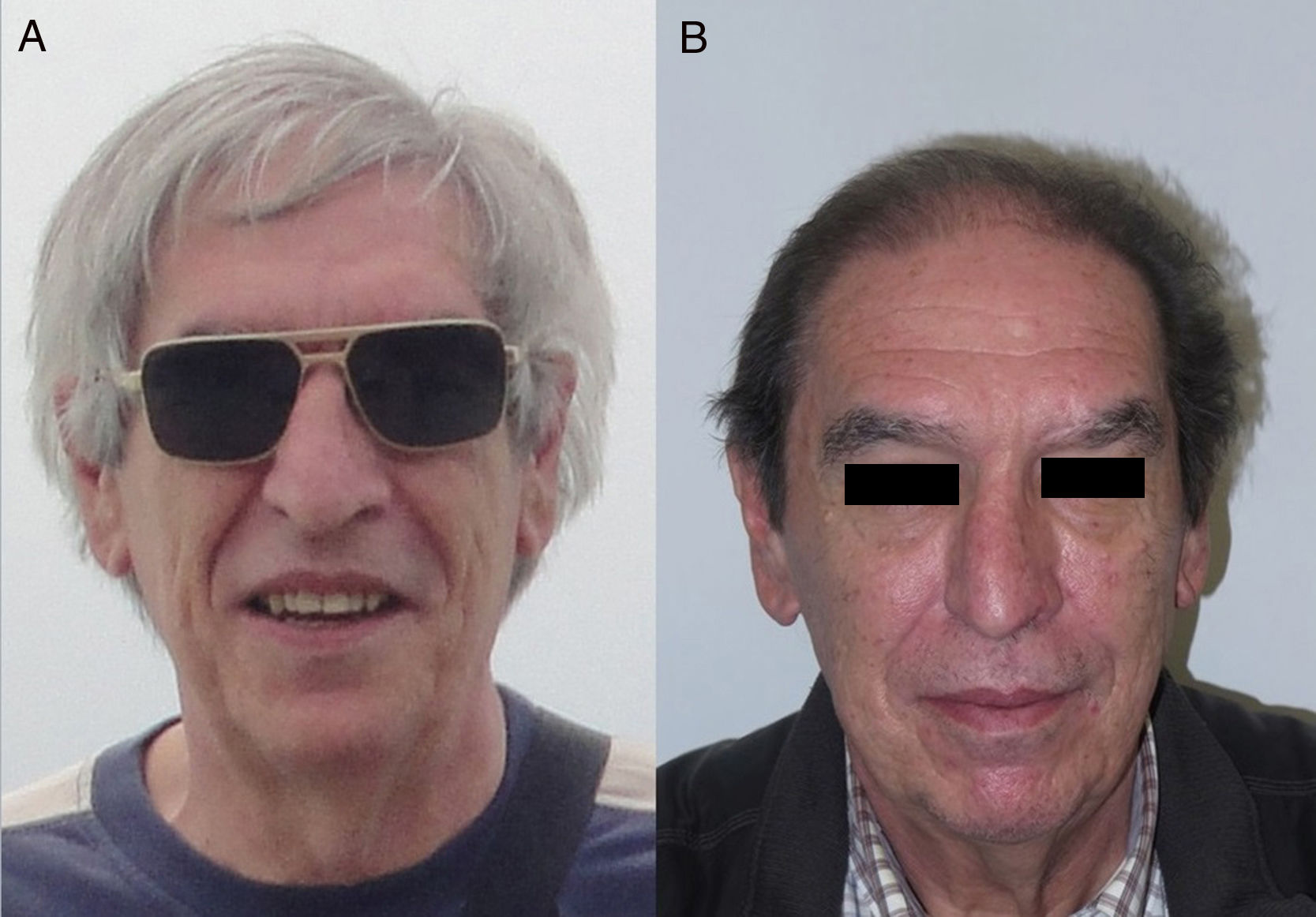
Anti-PD-1/PD-L1/CTLA-4: Nivolumab, Pembrolizumab/Atezolizumab, Durvalumab/IpilimumabPD-1, PD-L1, and CTLA-4 inhibitors are a heterogeneous group of drugs familiar to dermatologists because they are used in the treatment of metastatic melanoma. In the case of ipilimumab, the onset of vitiligo-like hypopigmentation is common (particularly in patients with melanoma), and in initial studies, up to 14% of patients experienced hypopigmentation of hair of the scalp, eyebrows, or eye lashes.18,19 Subsequently, there have been 2 reports of poliosis with this class of drugs, 1 with ipilimumab20 and the other with pembrolizumab.21 However, the reverse effect seems more frequent with PD-1/PD-L1 inhibitors, as a series of 14 patients was recently published of scalp repigmentation after receiving treatment with 1 of these PD-1/PD-L1 agents for nonsmall cell lung cancer (Fig. 3).22 Of note is that 13 of the 14 patients were men.
Although alopecia arising from anti-PD-1 agents occurs in 1% to 2% of patients, there are no studies that adequately characterize this effect.23 With ipilimumab, the proportion of patients with alopecia is around 5.1% (ranging from 1.3% to 18.3% according to the series).9 Recently, a series of 4 cases has been published of patients during combined treatment with PD-1/PD-L1+ipilimumab, who developed a clinical picture indicative of alopecia areata (AA); 3 of them in plaque form and 1 case of alopecia universalis with nail involvement.23 Two cases of AA related to ipilimumab use have been reported.24,25 In 1 of them, AA was associated with extensive vitiligo and the authors argued that the presence of autoimmune phenomena could be a marker of longer survival.
Another anecdotal case involved a change to a persistent curly hair phenotype in a patient receiving treatment with nivolumab.26
BRAF Inhibitors: Vemurafenib, DabrafenibBRAF inhibitors were pioneers in targeted therapy for metastatic melanoma. These treatments have a complex safety profile, which has been improved through combination with MEK inhibitors.27 The effects of BRAF inhibitors on hair are varied and common. Alopecia, typically of the diffuse, nonscarring form of mild intensity (grade i-ii), is a common side effect, present in 8% in the first clinical trials of vemurafenib and up to 100% in a prospective study of 11 patients.28 However, the most reliable data probably are derived from a cohort of 3219 patients treated with vemurafenib, up to 25% of whom had some form of alopecia.29 This rate was similar to that reported in a metaanalysis (23.7%).9 We have less extensive data on the percentage of dabrafenib-treated patients who develop alopecia. A series of 119 patients showed an incidence of 14%,30 which in the aforementioned metanalysis increased to 18.9%.9 Furthermore, of note is that this type of alopecia is often accompanied by changes in hair morphology (to curly phenotype), hair thickness, and hair color (typically grey hair may appear or, occasionally, repigmentation is reported).27,31 This occurs in 16.7% of patients with vemurafenib and 13% to 60% of those with dabrafenib (usually in mild form), depending on the series.30,32 This set of findings has been denoted RASopathic alopecia by some authors.31 In addition, the appearance of interfollicular yellow dots in the scalp is characteristically observed in association with these changes.32 In most cases, these effects occur at the start of treatment, although recently a late-onset case has been reported.33
Finally, the appearance of seborrheic dermatitis of the scalp is not uncommon, and management can be challenging,34 with some of the most affected patients developing forms of pityriasis amiantecea.35
MEK Inhibitors: Trametinib, Cobimetinib, Binimetinib, SelumetinibAs we have already pointed out, MEK inhibitors are usually used in combination with BRAF inhibitors (vemurafenib and dabrafenib), not just to increase survival but to reduce side effects.26,27 Therefore, specific reports on their side effects on hair when used as monotherapy are limited. In one of the phase iii clinical trials that included 323 patients with trametinib, alopecia was reported in 19%, in almost all cases of grade i severity.36 According to a metaanalysis, the overall percentage of such events among patients treated with trametinib was close to 13.3%.9 In another small series of 11 patients, selumetinib was reported to cause alopecia in 9% of cases.37
However, the most important aspect of these drugs is, without doubt, their capacity to improve the safety profile of BRAF inhibitors, the agents with which they are almost always used in combination. Comparative studies between BRAF inhibitors alone and a combination of BRAF and MEK inhibitors show that most of the usual side effects occur at a lower frequency with combination therapy.26,30,37 This however is not the case with photosensitivity and keratosis pilaris, whose incidence may increase with combination therapy.27 With regards hair, the combination does seem to improve the safety profile, as shown in a comparative study in which the incidence of alopecia was compared for patients treated with dabrafenib (14%) and those treated with a combination of dabrafenib and trametinib (3.3%); the incidences of curly hair and grey hair was also compared (12.6% vs. 3.3%, respectively).30
Recently, a case of trichorrhexis nudosa was reported in a 14-year-old girl, associated with treatment with trametinib.38
KIT/BCR-ABL Inhibitors: Axitinib, Imatinib, Regorafenib/Bosutinib, Dasatinib, Nilotinib, PonatinibThis heterogenous class of drugs inhibit tyrosine kinases produced by c-kit, platelet growth factor receptors, and BCR-ABL fusion. They are mainly used for hematological malignancies, such as chronic myeloid leukemia, although they have a wide range of other indications.13 The most characteristic side effects of this group are associated with pigmentation disorders,19 given the role of c-kit in melanocyte physiology.13 Multiple patterns of skin hypopigmentation or depigmentation have been described with imatinib,19 with possible hair involvement39,40 (sometimes in form of grey hair).41,42 These alterations are more common in patients with dark phototypes and they usually respond to decreased doses or remit on suspending the drug.43 Several cases have also been reported of leukotrichia caused by dasatinib, whether or not associated with skin hypopigmentation,44–46 and by regorafenib.47 The opposite effect (repigmentation), although less frequent, has also been reported both in skin, mucosas, and nails19,43 and in hair, in 2 different series with imatinib: a series of 9 cases out of 133 treated patients (median duration, 5.7 months)48 and another of 8 cases (out of 58).49
Another adverse effect on hair observed frequently is alopecia, particularly in women. Regorafenib is the agent in this class with the highest rate of alopecia (23.7%).9 Although nilotinib also shows high rates of alopecia (6%-8% according to pivotal studies, with one metaanalysis finding an incidence as high as 15.9%),43,50 this effect is not well characterized.13 In one case, the onset of complete diffuse alopecia of an inflammatory nature is described in detail (affecting the scalp and eyebrows but not the eyelashes, body hair, or nails) in a female patient, associated with an eruption of multiple flesh-colored follicular papules on the trunk, several weeks after starting nilotinib.51 In addition, the event coincided with a recent postpartum period. Given that alopecia in these cases does not resemble the usual form of onset, the authors proposed the term nilotinib-induced alopecia. To date, there have not been any further reports on this specific type of alopecia. Imatinib can also cause alopecia, which is reported in 6.6% of patients,9 although complete alopecia is uncommon.52 In a comparative study of axitinib and sorafenib (both of which are indicated for metastatic renal cancer), alopecia was reported in 4% of patients treated with axitinib.53 Dasatinib has an alopecia rate of approximately 7.8%, although this may vary from 3% to 19.1% according to the series.9
In addition to alopecia and pigmentation disorders, other types of side effects that impact hair can be observed. Keratosis pilaris caused by nilotinib is common54; this may be atrophic and is described as associated with plaque AA.55 Of note also is that lichenoid reactions are common in this group of drugs and, at times, this may affect hair in the form of lichen planopilaris.56 Moreover, with some of these drugs (nilotinib, dasatinib, and ponatinib), cases have been reported in which the clinical manifestations could be mixed, typically combining a finding of lichen planopilaris with skin eruptions such as keratosis pilaris, reminiscent of Graham-Little syndrome.57 In addition, anecdotally, there have been reports of imatinib associated with a case of madarosis in a pediatric patient58 and of nilotinib with a case of perforating folliculitis.59
VEGF/VEGFR Inhibitors: Bevacizumab, Pazopanib, Sorafenib, Sunitinib/Lenvatinib, RamucirumabDrugs that belong to the class of VEGF/VEGFR inhibitors are also called multikinase inhibitors because, in addition to inhibiting activity of the intracellular portion of the VEGF receptor, they act on many other tyrosine kinases either directly or indirectly.13 For this reason, they exhibit a wide range of adverse effects and many of these coincide with adverse effects characteristic of other classes of drug.
The most frequent adverse effect on hair is alopecia.13 Several studies support this affirmation: different metaanalyses cite the percentage of patients with alopecia at 6% (5%-21%) with sunitinib,60 10% (3.3%-26.8%) with bevacizumab,9 12.3% (9%-16%) with pazopanib,9 and 25.5%-29% with sorafenib,61 the drug with the highest percentage of affected patients. Furthermore, sorafenib-associated alopecia has its own distinctive characteristics. Onset usually occurs between week 3 and week 15 of treatment and may at times be accompanied by slow growth of facial hair.62 Another study reported that up to 19% of patients experienced loss of body hair, not always associated with alopecia of scalp hair.63 Management is complex, as there is no specific treatment available, but the disorder is not usually permanent and the patient regains hair density once treatment is complete (and sometimes even before completion). When hair regrows, it may appear brittle and curly.64 Other authors have reported that the event in some patients may have characteristics that overlap with AA.65 With regards the VEGFR inhibitors lenvatinib and ramucirumab, limited data are currently available. Ramucirumab, according to a metaanalysis of 6 randomized clinical trials, does not show an increase in the proportion of patients with alopecia compared with placebo,66 whereas with lenvatinib, 11% of patients had alopecia in a phase iii clinical trial with 261 patients.67
Through direct or indirect activity on c-kit, these drugs are also often associated with skin and hair pigmentation disorders.13 In this case, sunitinib is typically the most widely implicated drug,62 with depigmentation of the hair, eyebrows, and eyelashes in up to 8% to 14% of cases.42,68–70 Onset usually occurs after 5 to 6 weeks of treatment, with hair acquiring a greyish color. The effect usually remits within 2 to 3 weeks of finishing treatment, and so it is possible that there are intermittent cases of depigmentation, depending on the periodicity of treatment (due to transient inhibition of c-kit).62,69 Although this effect is usually attributed to sunitinib, in some series, the proportion of hair depigmentation with pazopanib is even higher, reaching 38%71 at high doses, with rapid onset72 and an odds ratio of 4.54.73
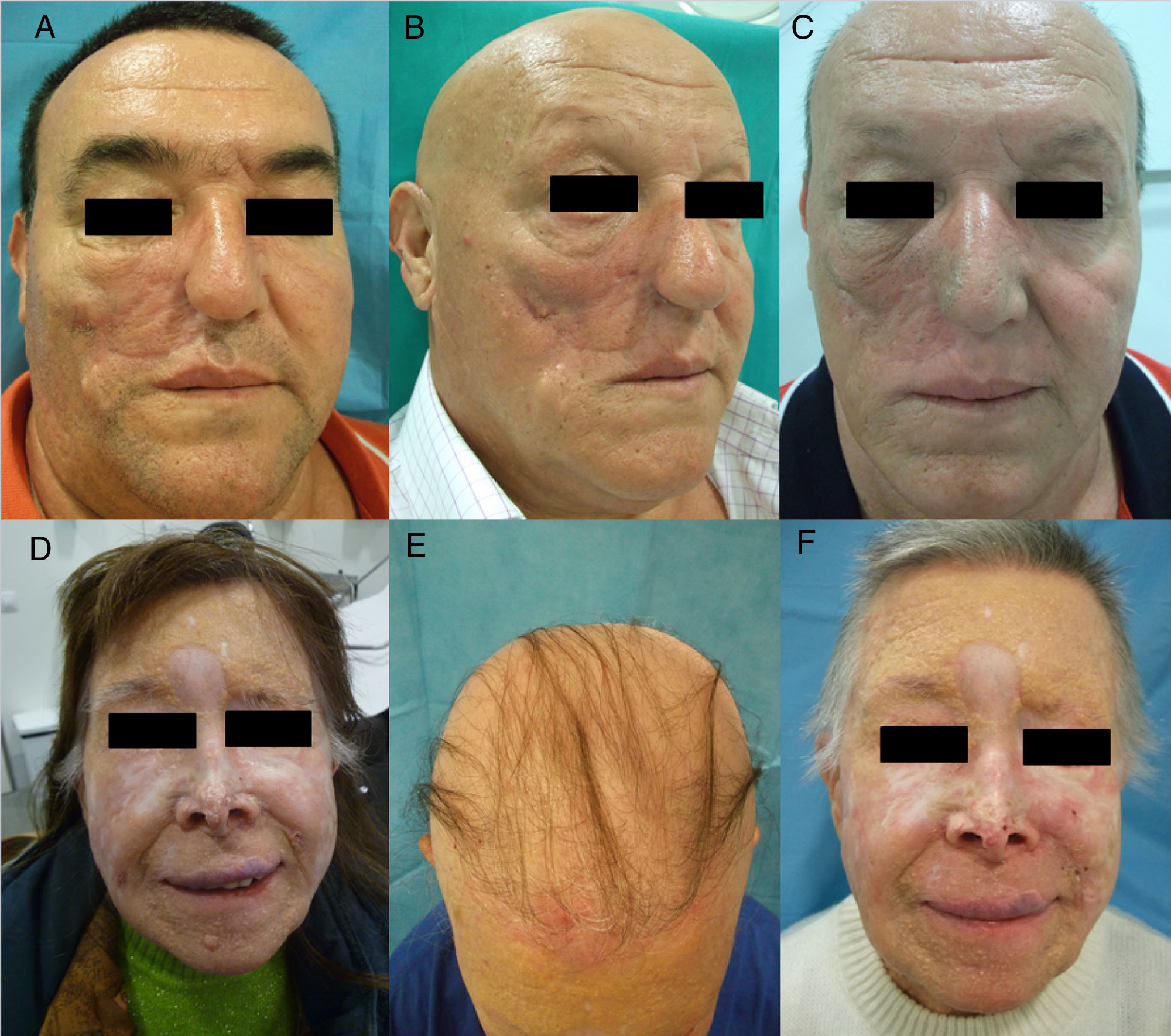
Hedgehog Inhibitors: Vismodegib, Sonidegib, ErismodegibHedgehog inhibitors are primarily indicated for advanced and metastatic basal cell carcinoma.31 In this case, alopecia is, without doubt, the most frequent adverse effect involving hair (Fig. 4). The rates are very high, reaching 63% in the first pivotal clinical trials with vismodegib.74 In fact, vismodegib is the cancer drug with the highest rate of alopecia according to a comparative metaanalysis.9 Furthermore, of note is that 10%-14% have grade ii alopecia (>50% hair loss).74 Another important study published recently of 694 patients with advanced and metastatic basal cell carcinoma treated with vismodegib reported a percentage of patients with alopecia of 62%, with the event occurring constantly throughout the first 12 months of treatment: it was responsible for withdrawal from treatment in 3% of patients.75 Of note is that this alopecia resolves spontaneously on stopping treatment (usually during the first year),75 4 cases have been reported of persistent alopecia (mean duration of follow-up of 15 months).76 In addition, other authors report 2 cases in which spontaneous improvement in this process during treatment was accompanied by treatment failure, and they speculate whether this could be an early marker of therapeutic failure and disease progression.77 Alopecia associated with sonidegib, although not as common, is still present in more than 10% of cases.78 The Hedgehog pathway shows high signaling levels in growing follicles, which would explain the high rates of alopecia when this pathway is inhibited.79 In fact, agonists of the Hedgehog pathway have been studied as potential treatments for androgenic alopecia (and have been ruled out due to their numerous adverse effects).80 A recent study characterized in detail the pattern of alopecia associated with these drugs in 5 patients (4 with vismodegib and 1 with sonidegib/erismodegib).81 In all cases, the authors describe the cases as a diffuse, noninflammatory alopecia (without an androgenic pattern), with slow and gradual onset, and regrowth of increasingly fine and weak hair. This process occurred between 2 and 9 months (median of 3.1 months) of treatment and often affected body hair and eyebrows (in 4 out of 5 cases) and nails (3 out of 5). In no case were signs of AA observed. The only histopathological findings were an increase in the telogen to anagen ratio, consistent with telogen effluvium.
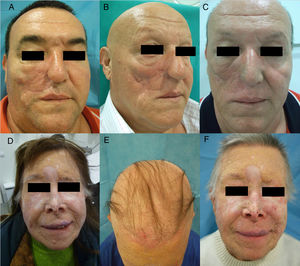
Diffuse alopecia caused by vismodegib (Hedgehog inhibitor) in patients included in the STEVIE study. A, Male patient before starting treatment. B, After 8 months of treatment. C, At 3 months after discontinuation of treatment. D, Female patient before starting treatment. E, After 9 months of treatment. F, At 3 months after discontinuation of treatment. Courtesy of Dr. V. Ruiz-Salas.
Another curious effect associated with vismodegib treatment, reported once anecdotally, is trichodysplasia spinulosa.82
Proteasome Inhibitors: Bortezomib, Carfilzomib, IxazomibProteasome inhibitors are drugs used mainly in the treatment of hematological malignancies. Alopecia caused by bortezomib is considered uncommon,9 with incidences ranging from 0.4% to 10.9%.83,84
mTOR Inhibitors: Everolimus, Temsirolimus, SirolimusmTOR inhibitors are drugs with many wide-ranging indications that generally have limited impact on hair.31,85 These agents are considered as an uncommon cause of hair loss.9 The percentage of patients with alopecia is 5.3% (1.9%-14.3%) with everolimus and 5.2% (0.9%-25.9%) with temsirolimus.9 With sirolimus, the prevalence of alopecia, usually mild and diffuse, is 9%, and occasionally, it may also affect body hair.86 The following effects have also been reported for sirolimus: hypertrichosis (face and body), 2 cases of AA, and folliculitis of the scalp in up to 25% of patients.86
PI3K Inhibitors: Idelalisib, Copanlisib, DactolisibPI3K is a promising target for hematological malignancies and brain tumors, with limited data on capillary reactions. The presence of high PI3K activity in hair follicle cells would seem to indicate that inhibition of this pathway could lead to a high percentage of alopecia.87 In fact, in murine models with dactolisib, alopecia was the most frequently observed adverse effect.88 However, the data available to date with idelalisib (the PI3K inhibitor for which most clinical experience is available) suggest that the percentage of patients with alopecia is low.89
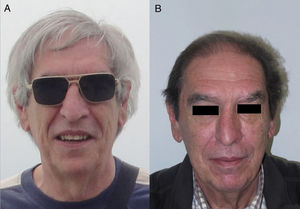
Anti-CD52: AlemtuzumabAlemtuzumab is an anti-CD52 agent with a range of indications. Characteristically, it is associated with several autoimmune adverse effects.90 In this context, there have been reports of AA associated with alemtuzumab, both in plaque form and the universalis form, which can be accompanied by other events of autoimmune origin (Fig. 5).91 Furthermore, the rate of alopecia is 5.3% (0.7%-29.4%).9
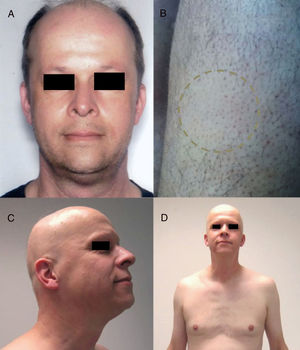
Alopecia areata (AA) universalis caused by alemtuzumab (anti-CD52 agent). A, Appearance before treatment. B, Initial plaques of AA on the legs. C and D, Final phase of AA universalis.
Source: Van der Zwan et al.91.
ALK inhibitors are indicated mainly for nonsmall cell lung cancer. The rate of alopecia caused by crizotinib is 8% (4.9%-13.2%).9,92 Data for other ALK inhibitors are limited, although a case of diffuse alopecia caused by alectinib has been reported in a patient who had already received crizotinib without alopecia occurring.93
HDAC Inhibitors: Belinostat, Panobinostat, Romidepsin, VorinostatThere are limited data on HDAC inhibitors to date. A study of 6 patients with advanced cutaneous T-cell lymphoma who received treatment with vorinostat detected 2 cases of mild alopecia and 1 case of moderate alopecia, with no other effects of note on hair.94
RANKL Inhibitors: DenosumabDenosumab is the main drug in this class and is indicated for the treatment of osteoporosis and bone metastases. Alopecia has not been reported among the common adverse effects after 10 years of use.95 One case of AA universalis has been reported related to use of denosumab after only 2 weeks of treatment.96 The authors discussed whether the fact that RANKL belongs to the TNF-α family might bear some relation on the appearance of this effect, given that anti-TNF-α agents have been frequently associated with onset of paradoxical AA.
PARP Inhibitors: Olaparib, Rucaparib, Veliparib, Talazoparib, IniparibPARP inhibitors are drugs in development mainly for metastatic ovarian cancer. In a clinical trial of 306 patients, it was shown that there was no increase in the proportion of patients with alopecia in the veliparib arm compared with placebo.97 Another trial also showed a statistically significant decrease in the percentage of patients with alopecia who received veliparib/temozolomide instead of the traditional chemotherapy regimen with carboplatin/paclitaxel.98 Olaparib was also able to reduce the rate of alopecia when combined with traditional chemotherapy in comparison with a chemotherapy-only regimen.99
Anti-CD30: BrentuximabBrentuximab is any anti-CD30 agent indicated mainly for refractory Hodgkin lymphoma.100 The proportion of patients with alopecia after receiving brentuximab is 14% (9.9%-19.6%).9 Furthermore, anecdotally, there is a case report of complete hair repigmentation in a 72-year-old man after 6 months of treatment with brentuximab.101
ConclusionsHair-related side effects are common with new targeted therapies used in cancer (Table 2). All dermatologists should be aware of the most important ones to enable appropriate management of these patients, who are seen increasingly frequently in our clinics (Table 3). Several different types of hair reactions have been reported depending on the pharmacological class. These can be associated with the morphology of the hair, such as characteristic trichomegaly with EGFR inhibitors. Pigmentary changes are also frequent and range from typical depigmentation with c-kit or VEGF/VEGFR inhibitors to surprising repigmentation associated with PD-1/PDL-1 inhibitors. Even the most typical reactions, such as alopecia with Hedgehog inhibitors, show specific and differentiated characteristics according to the drug used. Other effects reported less often include entities that resemble classic hair diseases, such as AA, lichen planopilaris, and erosive pustular dermatosis of the scalp. Although the rapid advance of targeted therapy does not afford any rest, this review may serve as an initial guide for consultation and study of adverse effects on hair related with these drugs so far.
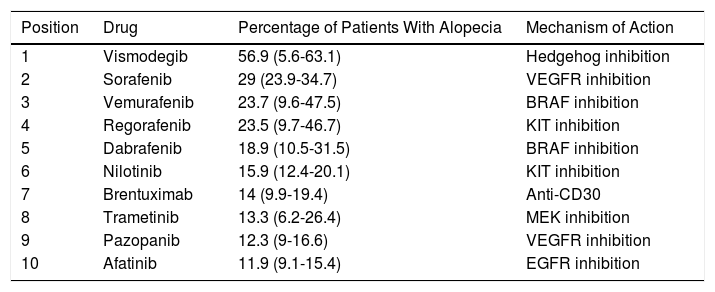
The 10 Drugs With Highest Incidence of Alopecia and Their Mechanism of Action.
| Position | Drug | Percentage of Patients With Alopecia | Mechanism of Action |
|---|---|---|---|
| 1 | Vismodegib | 56.9 (5.6-63.1) | Hedgehog inhibition |
| 2 | Sorafenib | 29 (23.9-34.7) | VEGFR inhibition |
| 3 | Vemurafenib | 23.7 (9.6-47.5) | BRAF inhibition |
| 4 | Regorafenib | 23.5 (9.7-46.7) | KIT inhibition |
| 5 | Dabrafenib | 18.9 (10.5-31.5) | BRAF inhibition |
| 6 | Nilotinib | 15.9 (12.4-20.1) | KIT inhibition |
| 7 | Brentuximab | 14 (9.9-19.4) | Anti-CD30 |
| 8 | Trametinib | 13.3 (6.2-26.4) | MEK inhibition |
| 9 | Pazopanib | 12.3 (9-16.6) | VEGFR inhibition |
| 10 | Afatinib | 11.9 (9.1-15.4) | EGFR inhibition |
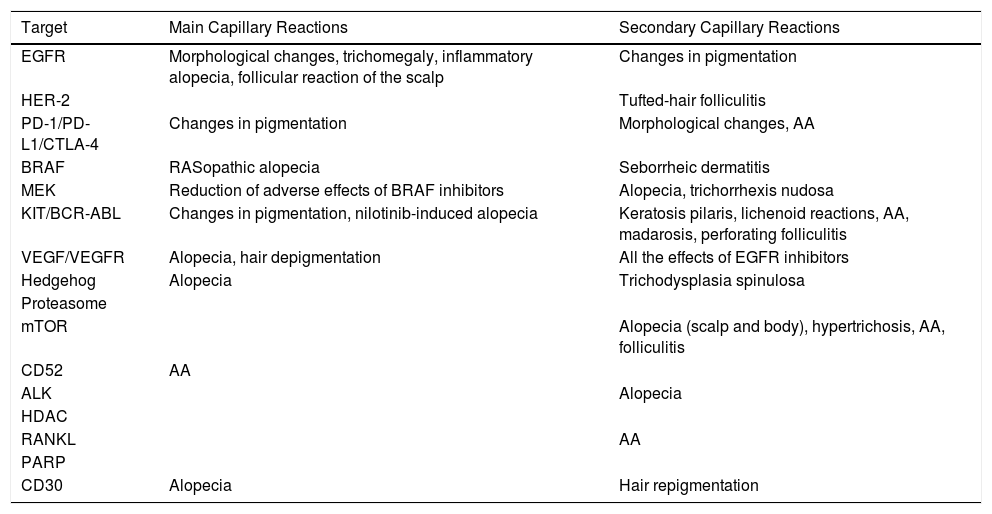
Summary of the Main and Secondary Capillary Reactions of Targeted Cancer Therapy by Pharmacological Class.
| Target | Main Capillary Reactions | Secondary Capillary Reactions |
|---|---|---|
| EGFR | Morphological changes, trichomegaly, inflammatory alopecia, follicular reaction of the scalp | Changes in pigmentation |
| HER-2 | Tufted-hair folliculitis | |
| PD-1/PD-L1/CTLA-4 | Changes in pigmentation | Morphological changes, AA |
| BRAF | RASopathic alopecia | Seborrheic dermatitis |
| MEK | Reduction of adverse effects of BRAF inhibitors | Alopecia, trichorrhexis nudosa |
| KIT/BCR-ABL | Changes in pigmentation, nilotinib-induced alopecia | Keratosis pilaris, lichenoid reactions, AA, madarosis, perforating folliculitis |
| VEGF/VEGFR | Alopecia, hair depigmentation | All the effects of EGFR inhibitors |
| Hedgehog | Alopecia | Trichodysplasia spinulosa |
| Proteasome | ||
| mTOR | Alopecia (scalp and body), hypertrichosis, AA, folliculitis | |
| CD52 | AA | |
| ALK | Alopecia | |
| HDAC | ||
| RANKL | AA | |
| PARP | ||
| CD30 | Alopecia | Hair repigmentation |
The authors declare that they have no conflicts of interest.
Please cite this article as: Mir-Bonafé JF, Saceda-Corralo D, Vañó-Galván S. Reacciones capilares de las nuevas terapias diana dirigidas contra el cáncer. Actas Dermosifiliogr. 2019;110:182–192.