While there are questionnaires for evaluating the effects of skin cancer on patient quality of life, there are no specific questionnaires available in Spanish for evaluating quality of life in patients with actinic keratosis. The aim of this study was to translate and culturally adapt the Actinic Keratosis Quality of Life (AKQoL) questionnaire into Spanish.
Patients and methodsThe original questionnaire was translated into Spanish following the guidelines for the cross-cultural adaptation of self-report measures. Several measures of general reliability and validity were calculated, including Cronbach α for internal consistency and the Spearman rank-order correlation coefficient and a Bland-Altman plot for test-retest reliability. To test concurrent validity, we used the Pearson correlation coefficient to measure the correlation between AKQoL and Skindex-29 scores.
ResultsThe final version of the questionnaire was administered to 621 patients with actinic keratosis, who scored a mean (SD) of 5.25 (4.73) points (total possible score, 0-25). The Cronbach α reliability coefficient analysis was 0.84. The correlation between the mean (SD) score on the Skindex-29 (1.87 [4.07]) and on the AKQoL (1.97 [2.98] was 0.344 (P=.002, Spearman's rho), with a proportion of shared variance of 11.8%.
ConclusionsThe translation, cross-cultural adaptation, and validation of the original AKQoL produced a reliable, easily understandable questionnaire for evaluating the impact of actinic keratosis on the quality of life of patients in our setting.
Actualmente existen cuestionarios para valorar el impacto en la calidad de vida en pacientes con cáncer cutáneo. Sin embargo, no se cuenta con un instrumento específico aplicable a casos con queratosis actínica. El objetivo de este estudio ha sido la traducción y adaptación transcultural del cuestionario Actinic Keratosis Quality of Life (AKQoL) al español.
Pacientes y métodosEl cuestionario original se tradujo al español de acuerdo con el modelo metodológico para la adaptación transcultural de medidas autoadministradas. Se han calculado diversos índices para la fiabilidad general y la validez, incluyendo el coeficiente alfa de Cronbach y test-retest (correlación de rango de Spearman y prueba de Bland-Altman), mientras que para la validez concurrente se utilizó el coeficiente de correlación de Pearson entre los resultados de los cuestionarios AKQoL y Skindex-29.
ResultadosLa versión final se administró a un total de 621 sujetos con queratosis actínica con una puntuación media ±desviación estándar de 5,25±4,73 (0 a 25 puntos). El análisis de fiabilidad mostró una alfa de Cronbach de 0,84. La correlación entre el Skindex-29 (1,87±4,07) y AKQoL (1,97±2,98) fue de 0,344 (p=0,002; rho de Spearman), con una proporción de varianza compartida del 11,8%.
ConclusionesMediante los distintos procesos de traducción, adaptación transcultural y validación en distintos grupos hemos generado una herramienta comprensible y fiable para valorar el impacto de la queratosis actínica en la calidad de vida de pacientes de nuestro entorno.
The concept of health-related quality of life (HRQOL) encompasses various dimensions, such as perception of health, level of independence, and interaction between a person and his or her environment.1–3 Although there is no widely accepted definition of HRQOL in the literature,4 the growing interest in this concept is undeniable. HRQOL assessment allows clinicians and researchers to evaluate the impact of disease on the well-being of patients and has become an important consideration when evaluating the outcomes of diverse medical interventions.
In the field of dermatology, concerns about aesthetic alterations, which are chronic in many cases, have led clinicians to use different tools to measure the impact of various skin diseases on their patients’ lives and their interaction with the environment.
Actinic keratosis (AK) is an incipient form of superficial squamous cell carcinoma (SCC) that typically presents as multiple lesions in areas of long-term exposure to UV radiation, generally from the sun but also from tanning devices. The condition is thus highly prevalent (affecting over 20% of individuals older than 60 years) and is associated with a risk of recurrence due to field cancerization (presence of subclinical lesions in photo-damaged skin underlying clinically evident lesions). Generally nonsurgical treatment is necessary to prevent progression to invasive forms of SCC.5 AK lesions present with varying grades of hyperkeratosis and manifest in multiple forms, ranging from slight scaling to highly adherent crusts. They frequently cause itching or a burning sensation.
Numerous scales are available to assess the impact of dermatologic conditions on QOL and they have been widely used in clinical practice and research. Examples are the Skindex-296 and the Dermatology Life Quality Index (DLQI).7 While other more specific instruments, such as the Skin Cancer Index8 (recently validated in Spanish9), are used to assess QOL in patients with nonmelanoma skin cancer, no specific questionnaires existed for AK until Esmann et al.10 developed the Actinic Keratosis Quality of Life questionnaire (AKQoL) in 2012.
Tools for evaluating QOL must, among other requirements, be understandable to the patient and this understanding is linked to sociocultural background. Most HRQOL scales, whether generic or specific to a disease and/or medical specialty, are created in English and only some have been translated and adapted for use in the Spanish population.11 When producing a new version of an HRQOL instrument, it is necessary to follow certain procedures to culturally validate the new instrument12 and ensure that it measures what it was designed to measure (validity); it must also be sufficiently reliable, i.e., offer the necessary stability, accuracy, and consistency for measuring a determined trait.13
ObjectiveThe aim of this study was to produce a linguistically and semantically equivalent version of the AKQoL in Spanish that would constitute a specific tool for evaluating the QOL of patients with AK in our country. We also wished to evaluate the psychometric properties of the Spanish AKQoL.
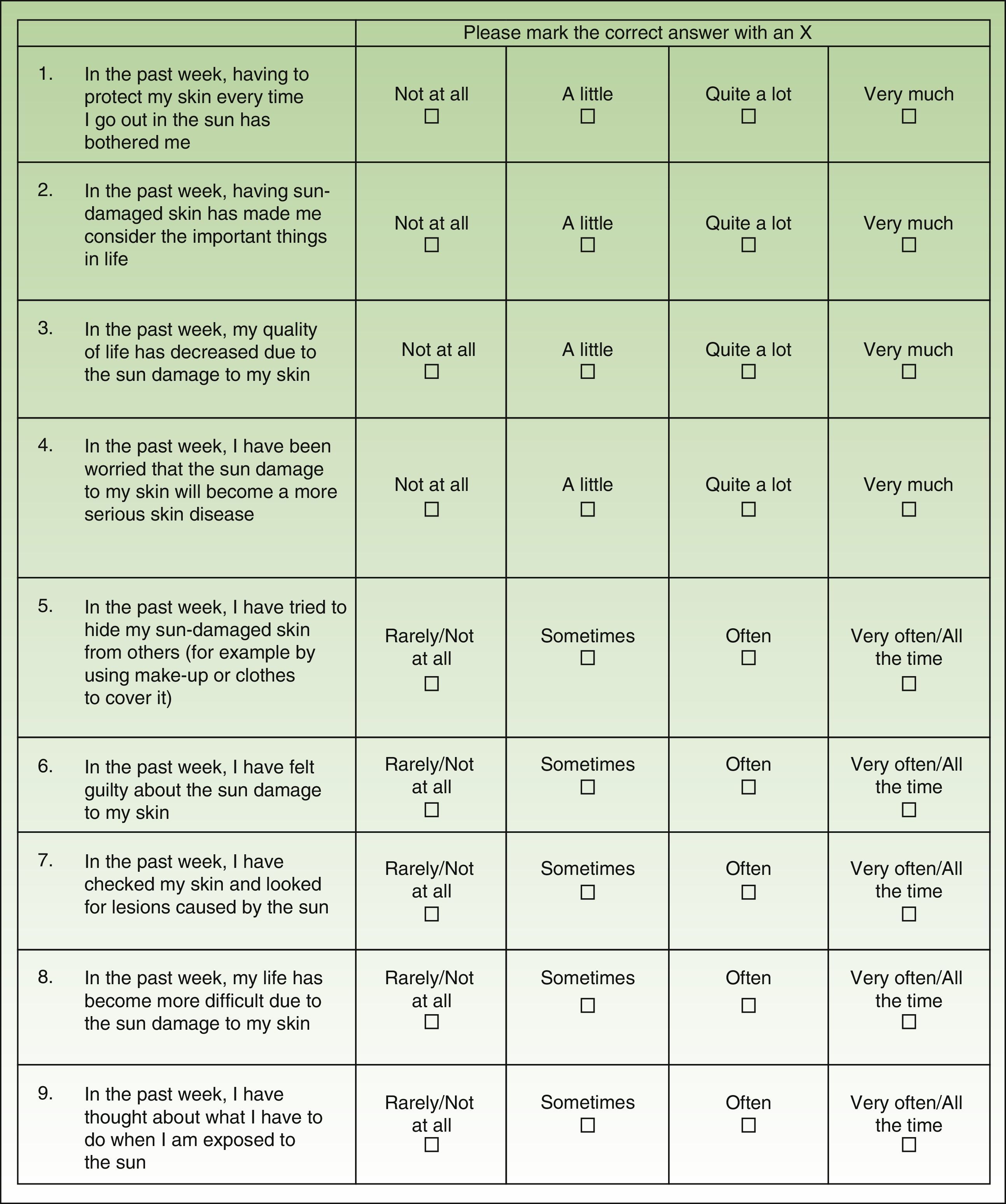
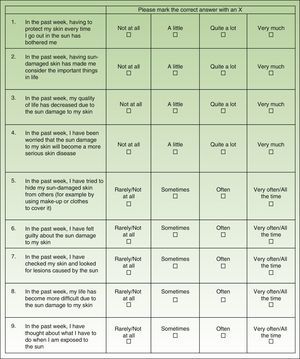
Material and MethodsThe AKQoL is a self-administered questionnaire, originally created in Danish, that evaluates different situations associated with the presence of AK lesions. It consists of 9 items scored on a 4-point scale ranging from 0 (rarely/not at all) to 4 (very much/all the time). It is composed of 3 domains, or subscales: function, which covers personal views of QOL, social life, and appearance (items 1, 8, and 9); emotions, which covers feelings such as fear, guilt, shame, worry, and irritation/bother (items 2, 4, and 6), and control over life in general (items 5 and 7). It also has a single global item (item 3). The total possible score thus ranges from 0 to 27, with higher scores indicating greater impairment of QOL.
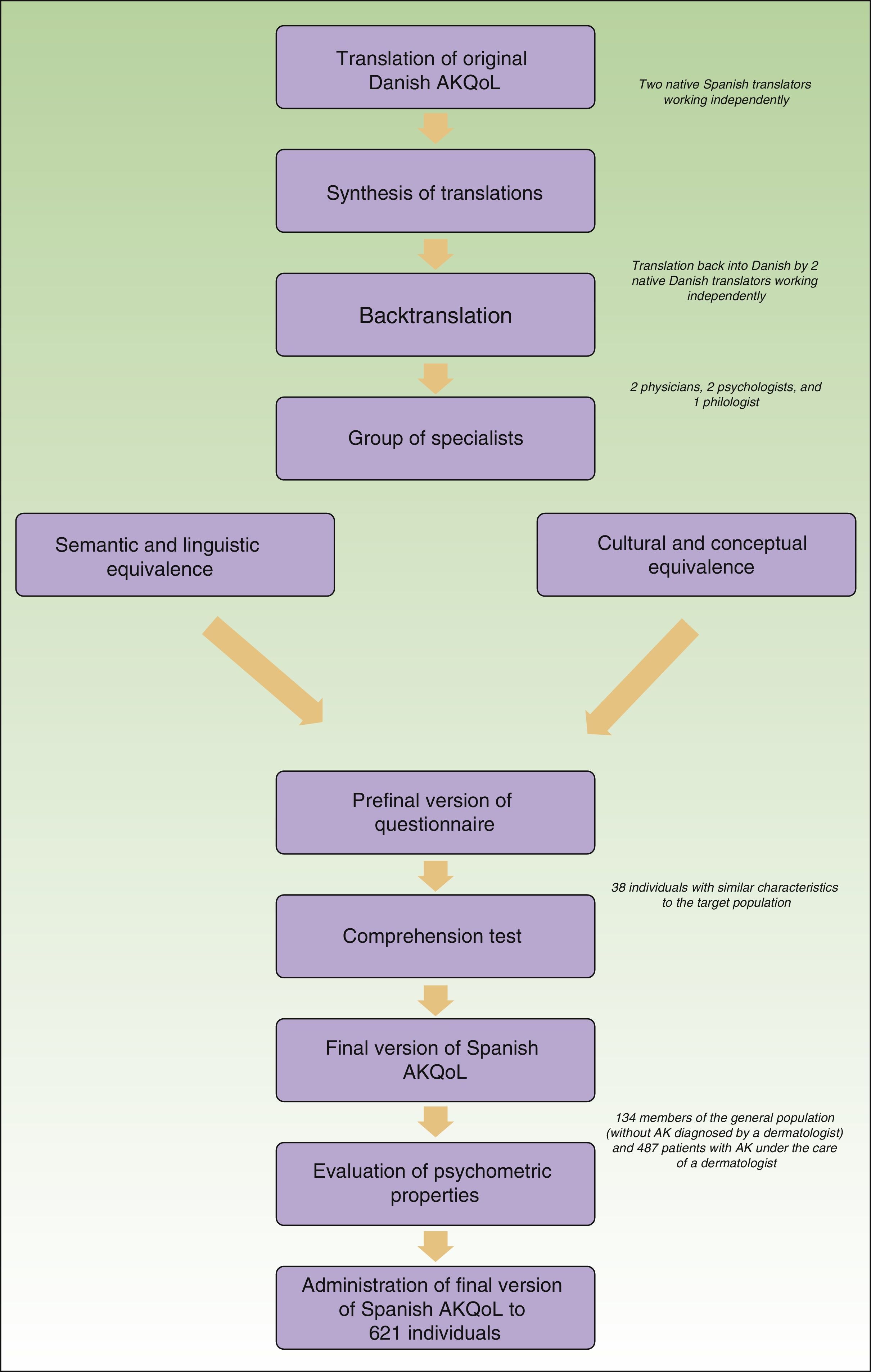
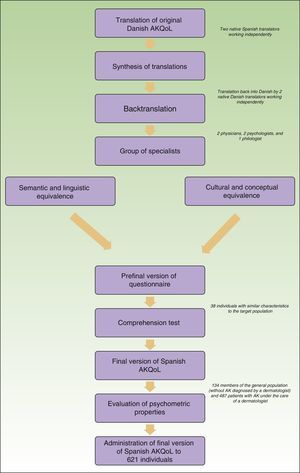
Adaptation of the AKQoLThe original Danish questionnaire was translated and culturally adapted in accordance with the methodological model for the cultural adaptation of self-administered questionnaires14 with the aim of maintaining semantic equivalence. The first step was the translation-backtranslation phase. The Danish questionnaire was translated into Spanish and the results were evaluated by a group of experts (Fig. 1). The Spanish questionnaire was then backtranslated (into Danish) by 2 native Danish translators and compared with the original. Once the preliminary version of the Spanish AKQoL had been agreed on, it was tested for understanding in a group of patients with comparable characteristics to the target population (38 elderly people with AK from the urban area of Barcelona) and then administered to a second group of volunteers without AK (people older than 18 years from the same geographic area) to evaluate test-retest reliability. The final version of the questionnaire was then administered to a third group of patients from a multicenter observational study to evaluate, among other factors, the impact of AK-related treatment on QOL.
All the participants who completed the questionnaire in the different phases were previously informed of the nature and objectives of the study and they all voluntarily agreed to participate and to have their data recorded. The study and study material were approved by the clinical research ethics committee at Hospital General Universitario Gregorio Marañón in Madrid, Spain.
Validation of the Spanish AKQoLA sample size of 10 cases per item has traditionally been used to calculate the necessary sample size to reliably estimate the internal consistency of a scale or questionnaire (using the Cronbach α coefficient). Alpha values of higher than 0.7 are considered to indicate acceptable consistency. That said, sample size calculations for Cronbach α using Monte-Carlo simulations have shown that errors are minimized when samples of over 300 cases are used.15 Thus, considering a statistical power of 90%, an alpha error of 0.05, and an expected Cronbach α of 0.81 (original scale),10 we calculated a necessary sample of 620 cases
Test-retest reliability was evaluated using the Spearman rank-order correlation coefficient and a Bland-Altman plot of scores for individuals who completed the questionnaire twice, 2 weeks apart. To assess construct validity, we calculated interitem and item-scale correlations for each item and created a correlation matrix for the 3 domains (function, emotions, and control).
To determine concurrent validity, we compared the Spanish AKQoL with another tool designed for a similar purpose (the Skindex 29) and measured the correlations between AKQoL and Skindex-29 scores using the Pearson correlation coefficient. To test the predictive validity of the questionnaire, we used 1-way analysis of variance to analyze differences among mean scores (for the overall questionnaire and the 3 domains) for members of the general population and patients with AK diagnosed by a dermatologist.
We also evaluated the reliability and quality of the data (distribution and score range) and floor and ceiling effects, which reflect the proportion of patients who obtain extreme scores. These effects are considered to be absent if under 15% of respondents achieve the lowest score and the highest score. All correlations were calculated using the Spearman rank-order correlation coefficient.
Results were calculated from the number of valid cases and expressed as mean (SD), extreme values (minimum and maximum), and frequencies and proportions.
A P value of less than .05 was considered statistically significant. All the results were analyzed using SPSS for Windows (version 22.0) and the R statistic program (version 2.15.0.)
ResultsThe translation, cultural adaptation, and backtranslation phase was completed without disagreements between the evaluators. The comprehension test was performed by administering the prefinal version of the questionnaire to 38 individuals (66.7% men and 33.3% women) with a mean (SD) age of 63.7 (8.34) years. Only 1 item required clarification, and this was not related to language.
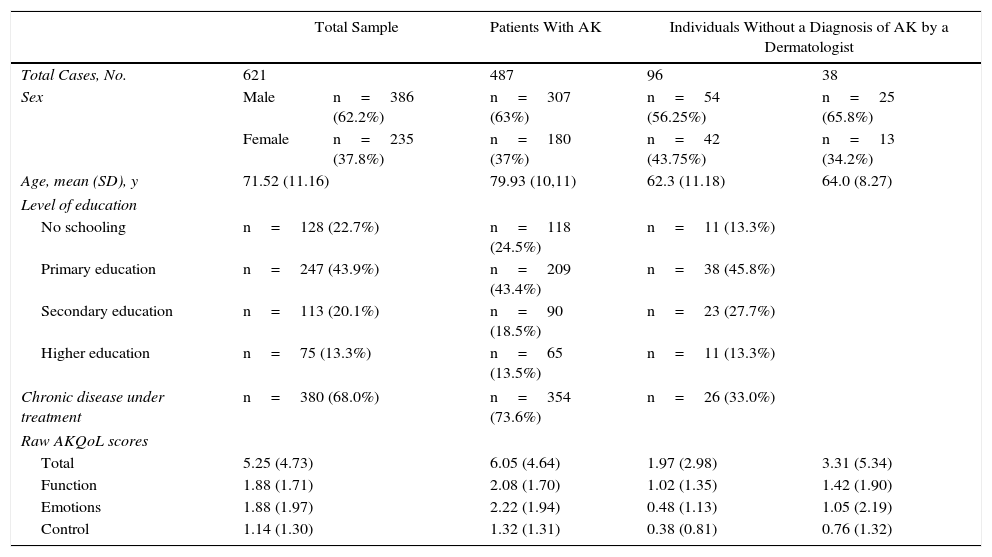
The final version of the AKQoL in Spanish was completed by 621 patients. The patients’ characteristics are summarized in Table 1.
Characteristics of Sample and Quality of Life Scores.
| Total Sample | Patients With AK | Individuals Without a Diagnosis of AK by a Dermatologist | |||
|---|---|---|---|---|---|
| Total Cases, No. | 621 | 487 | 96 | 38 | |
| Sex | Male | n=386 (62.2%) | n=307 (63%) | n=54 (56.25%) | n=25 (65.8%) |
| Female | n=235 (37.8%) | n=180 (37%) | n=42 (43.75%) | n=13 (34.2%) | |
| Age, mean (SD), y | 71.52 (11.16) | 79.93 (10,11) | 62.3 (11.18) | 64.0 (8.27) | |
| Level of education | |||||
| No schooling | n=128 (22.7%) | n=118 (24.5%) | n=11 (13.3%) | ||
| Primary education | n=247 (43.9%) | n=209 (43.4%) | n=38 (45.8%) | ||
| Secondary education | n=113 (20.1%) | n=90 (18.5%) | n=23 (27.7%) | ||
| Higher education | n=75 (13.3%) | n=65 (13.5%) | n=11 (13.3%) | ||
| Chronic disease under treatment | n=380 (68.0%) | n=354 (73.6%) | n=26 (33.0%) | ||
| Raw AKQoL scores | |||||
| Total | 5.25 (4.73) | 6.05 (4.64) | 1.97 (2.98) | 3.31 (5.34) | |
| Function | 1.88 (1.71) | 2.08 (1.70) | 1.02 (1.35) | 1.42 (1.90) | |
| Emotions | 1.88 (1.97) | 2.22 (1.94) | 0.48 (1.13) | 1.05 (2.19) | |
| Control | 1.14 (1.30) | 1.32 (1.31) | 0.38 (0.81) | 0.76 (1.32) | |
Abbreviations: AK, actinic keratosis; AKQoL, Actinic Keratosis Quality of Life.
a Values are presented as mean (SD) and total number (percentage) of patients and were all calculated using the total number of valid cases for each variable.
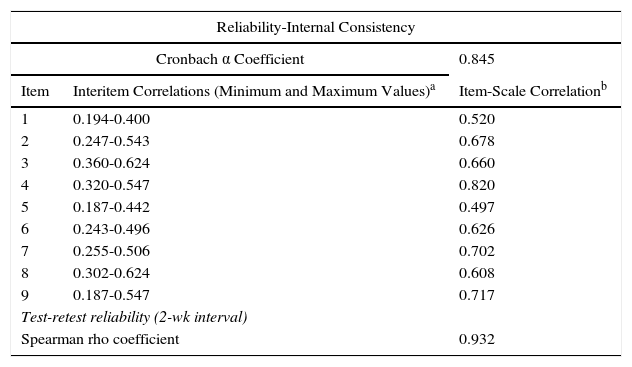
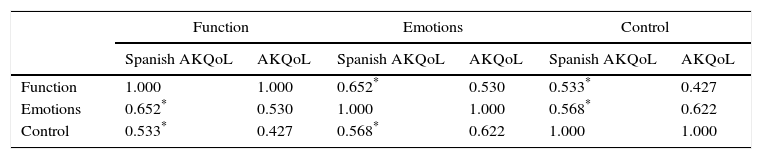
A Cronbach α of 0.84 was obtained for internal consistency. The interitem and item-scale coefficients were similar to those obtained for the original Danish scale. Correlation between the 3 domains of the Spanish AKQoL was also high, and the results were also similar to those of the original scale (Tables 2 and 3).
Psychometric Characteristics of the Spanish Actinic Keratosis Quality of Life Questionnaire.
| Reliability-Internal Consistency | ||
|---|---|---|
| Cronbach α Coefficient | 0.845 | |
| Item | Interitem Correlations (Minimum and Maximum Values)a | Item-Scale Correlationb |
| 1 | 0.194-0.400 | 0.520 |
| 2 | 0.247-0.543 | 0.678 |
| 3 | 0.360-0.624 | 0.660 |
| 4 | 0.320-0.547 | 0.820 |
| 5 | 0.187-0.442 | 0.497 |
| 6 | 0.243-0.496 | 0.626 |
| 7 | 0.255-0.506 | 0.702 |
| 8 | 0.302-0.624 | 0.608 |
| 9 | 0.187-0.547 | 0.717 |
| Test-retest reliability (2-wk interval) | ||
| Spearman rho coefficient | 0.932 | |
Correlationsa Between Domains in the Spanish Version of the Actinic Keratosis Quality of Life Questionnaire (AKQoL) and Results for the Original Questionnaire.
| Function | Emotions | Control | ||||
|---|---|---|---|---|---|---|
| Spanish AKQoL | AKQoL | Spanish AKQoL | AKQoL | Spanish AKQoL | AKQoL | |
| Function | 1.000 | 1.000 | 0.652* | 0.530 | 0.533* | 0.427 |
| Emotions | 0.652* | 0.530 | 1.000 | 1.000 | 0.568* | 0.622 |
| Control | 0.533* | 0.427 | 0.568* | 0.622 | 1.000 | 1.000 |
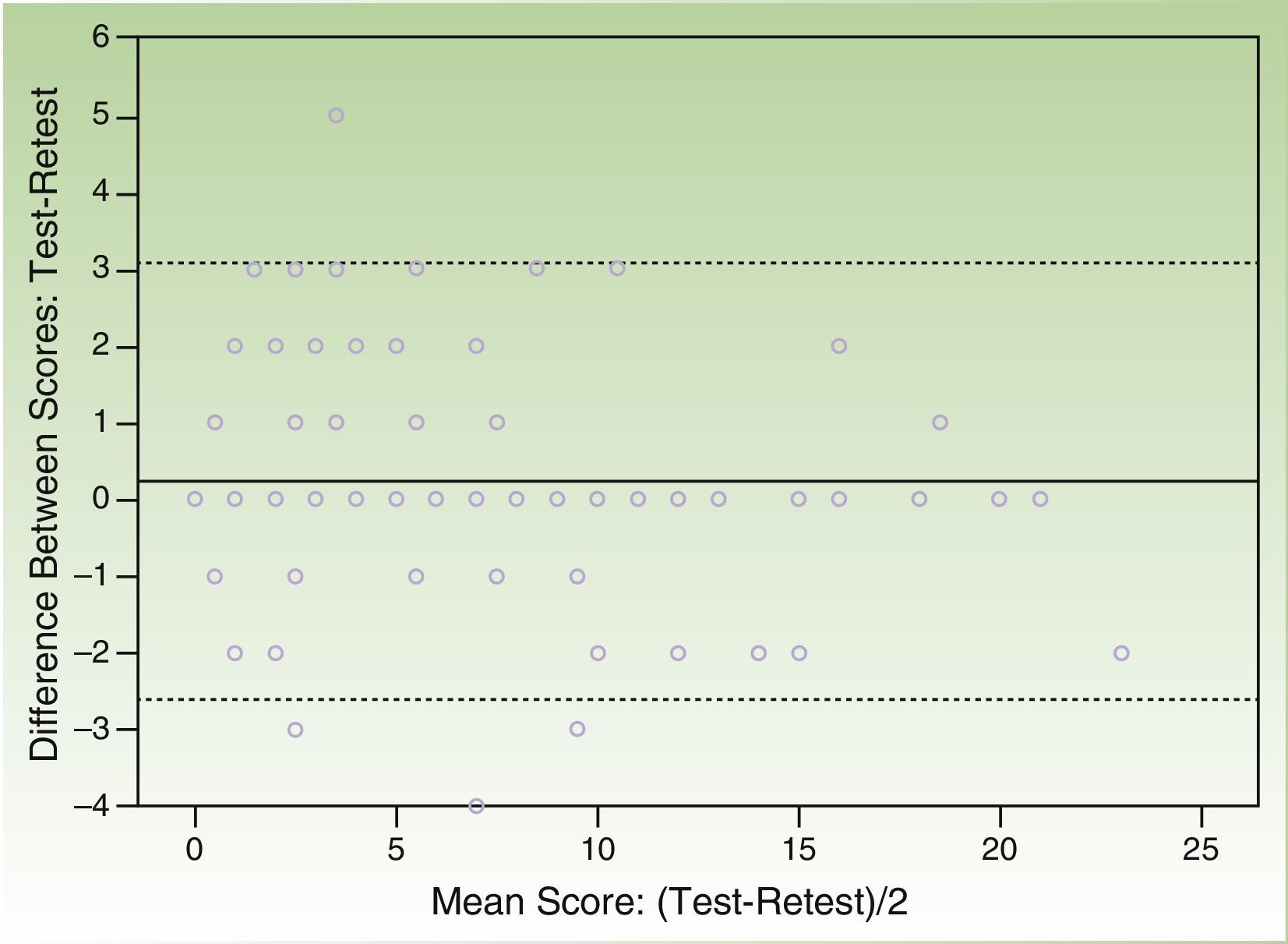
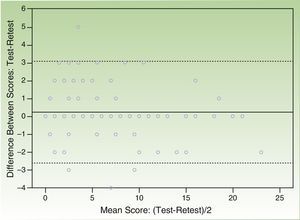
The correlation coefficient (Spearman rho) for test-retest reliability over 2 weeks was 0.932 and the Bland Altman plot of the scores from both questionnaires showed that the majority of individual differences were situated within the confidence limits of the mean (SD) difference of 0.22 (1.46) (95% CI, -2.64 to 3.08) points (Figs. 2 and 3).
Eighty participants rated their QOL using both the Skindex-29 and the AKQoL, and obtained mean scores of 1.87 (4.07) and 1.97 (2.98), respectively. The correlation between the scores was 0.344 (P=.002, Spearman rho), with an r2=0.118. Therefore, the proportion of shared variance between the 2 questionnaires was 11.8%.
Quality of LifeThe mean (SD) AKQoL score for the full sample was 5.25 (4.73). A minimum score of 0 was obtained in 110 cases (17.7%) and a maximum score of 25 was obtained in 1 case (0.2%). The mean scores per domain were 1.87 (1.7) for function (range, 0-8), 1.88 (1.97) for emotions (range, 0-9), and 1.14 (1.3) for control (range, 0-6).
Predictive ValidityPatients with AK diagnosed by a dermatologist scored significantly higher on the AKQoL than members of the general population (mean [SD] score of 6.05 [4.64] vs 2.35 [3.8], P<.0001).
DiscussionThe use of instruments to measure QOL and different aspects of health care is essential for both research purposes and for improving understanding of patient-related factors outside strictly clinical contexts. Conditions like AK are common reasons for consultation in routine practice, evidencing their impact. These conditions are chronic, affect visible parts of the body, frequently cause symptoms, and are invariably associated with local treatment-related adverse effects of varying severity.
We have produced a validated, culturally adapted questionnaire with adequate levels of internal consistency and high specificity for AK for use among patients with this disease in our setting.
According to the data in the literature,11 the AKQoL is the first specific HRQOL tool for patients with AK to be validated and adapted for use in Spanish. Not only is it semantically, conceptually, and culturally equivalent to the original questionnaire, but it also identifies differences between patients with AK diagnosed by a dermatologist.
Because AK is an incipient disease with a risk of malignancy, numerous aspects of its impact on QOL are not typically considered in other scales and questionnaires. De Troya-Martín et al.9 recently published the Spanish version of the Skin Cancer Index for measuring the QOL of patients with cervicofacial nonmelanoma skin cancer. The Skin Cancer Index has certain similarities to the AKQoL, not just because of the pathophysiological association between the 2 conditions, but also because of their possible psychological impact. The Skin Cancer Index, however, which has adequate psychometric properties, contains items that are specific to malignant lesions (e.g., evaluation of aspects such as metastasis and the physical, occupational, and social consequences of surgery) and not generalizable to AK.
Our evaluation of the psychometric properties of the Spanish AKQoL shows sufficient homogeneity between items and between each of the scales in relation to the original questionnaire. The low correlation with the Skindex-29, which is a generic scale with good psychometric properties, suggests that the AKQoL is highly specific for AK. A previous study of the AKQoL in patients in Denmark showed negative and nonsignificant correlations between raw scores on this questionnaire and other questionnaires, such as the EQ-5D.16 In addition, the mean scores obtained for the Spanish AKQoL and the Skindex-29 show much lower floor and ceiling effects for the AKQoL. Therefore, considering our results and those reported in the literature, the Spanish version of the AKQoL can be considered a complementary tool, as it measures distinct HRQOL factors.
One limitation of our study is that it was not possible to replicate the results of the factor analysis performed by Esmann et al.10 using a Rasch model. To compensate for this limitation, we applied nonparametric Spearman correlation tests to assess correlations between domains and compared our results to those of Esmann et al.
Apart from the procedures established for the validation of the AKQol and the questionnaire scores showing that AK has an impact on patient QOL, we would like to draw attention to the fact that women scored higher on average than men, although the difference was not statistically significant. Although these comparisons were not the object of our study, they do open the door to further research focusing on different aspects of QOL in patients with AK.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank all the health care professionals and patients who participated in the different cultural adaptation and validation phases of the study. We would also like to thank Dr Martí Gil for designing and helping with the psychometric evaluation and Dr Larios for providing editorial assistance during preparation of the manuscript. Both doctors work in the Medical Department of Clever Instruments SL in Barcelona.
Please cite this article as: Longo Imedio I,. Adaptación y validación de la versión española del cuestionario Actinic Keratosis Quality of Life (AKQoL). Actas Dermosifiliogr. 2016;107:474–481.