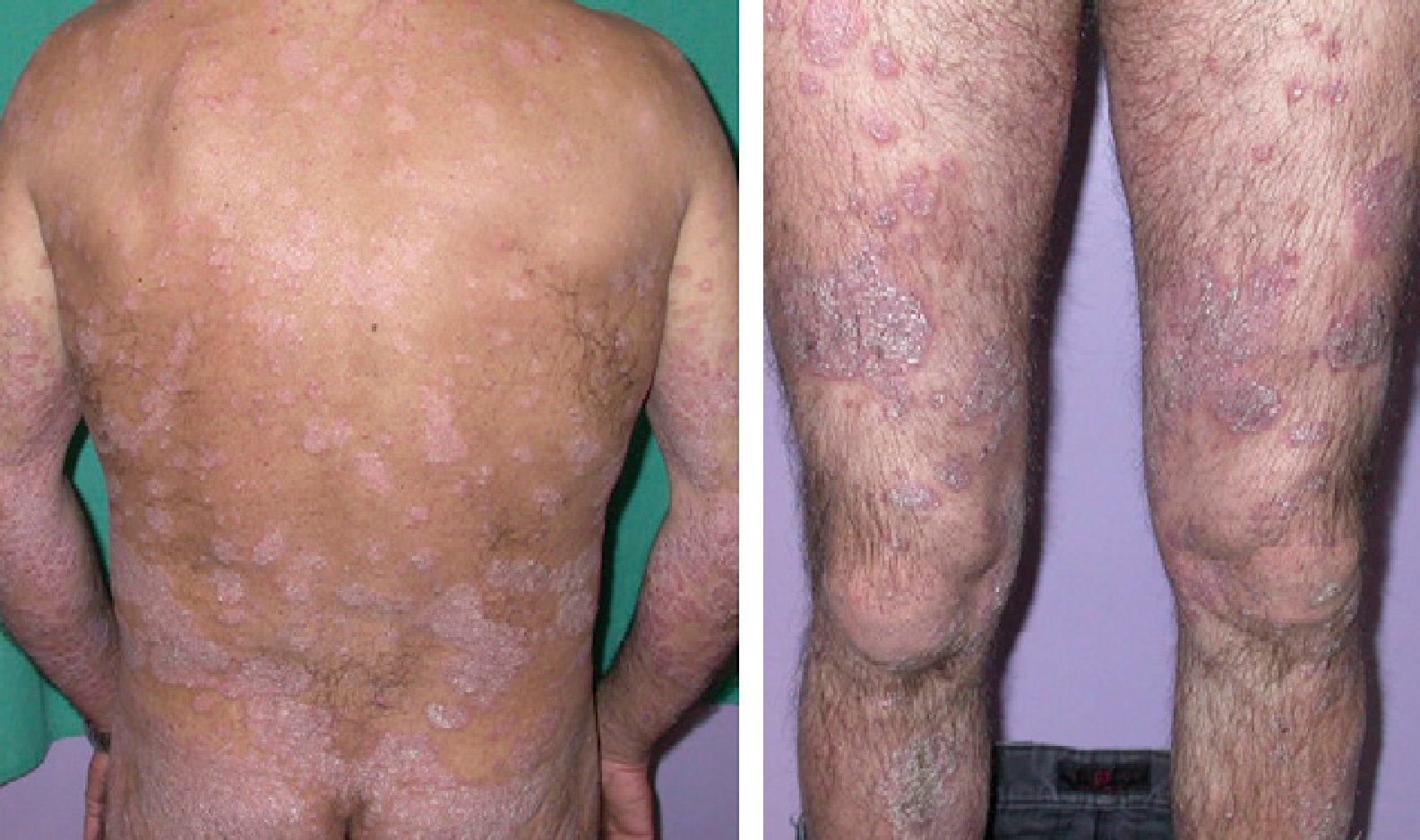
According to the literature, the use of tumor necrosis factor (TNF) inhibitors in patients with chronic hepatitis C infection is safe and effective. There have been no reports, however, of primary infection with the hepatitis C virus during treatment with a biologic agent. We report the case of a patient with long-standing moderate to severe psoriasis who developed acute hepatitis C while being treated with etanercept. Biologic therapy was continued and the infection was successfully treated with pegylated interferon, which achieved a sustained virologic response. Etanercept did not have a negative impact on disease outcome or on response to antiviral treatment.
El uso de agentes bloqueadores del factor de necrosis tumoral alfa (anti-TNFα) en pacientes con hepatitis C crónica ha sido descrito en la literatura en su conjunto como seguro y eficaz. Sin embargo, no se han descrito hasta la fecha casos de primoinfección por el virus de la hepatitis C ocurridos durante el tratamiento con un biológico. Presentamos un paciente con psoriasis moderada-severa de larga evolución que, estando en tratamiento con etanercept, sufrió una hepatitis C aguda. Sin suspender el fármaco anti-TNFα recibió tratamiento con interferón pegilado, con respuesta virológica sostenida. Etanercept no interfirió de forma negativa en la evolución de la enfermedad ni en la respuesta al tratamiento antiviral.
Tumor necrosis factor (TNF) antagonists have been used to treat a variety of conditions in patients with chronic hepatitis C virus (HCV) infection, as shown by numerous case reports in the literature. However, we have found no reports of primary HCV infection that occurred after treatment with an anti-TNF biologic had been started. We present such a case of primary HCV infection that occurred while the patient was on etanercept for psoriasis.
Case DescriptionA 45-year-old man with a history of smoking and anxiety had long-standing moderate-severe psoriasis that had been treated with corticosteroids and vitamin D analogs since 1999. In 2005 our department prescribed treatment with methotrexate at a weekly dose of 15mg. Response was good. However, symptoms worsened after 6 months, when a psoriasis area and severity index (PASI) of 23 was reached (Fig. 1). The advisability of treating this particular patient with a biologic agent was then considered. The pretreatment complete blood count and biochemistry showed normal values, and a chest radiograph was unremarkable. A tuberculin skin test and serology for HBV, HCV, and the human immunodeficiency virus were negative. Treatment with etanercept was begun in 2006 with the usual regimen of 50mg twice weekly for 3 months and followed with once-weekly administration thereafter. PASI 0 was reached after 6 months. Several relapses occurred between 2006 and 2009 on withdrawal of etanercept.
While the patient was on etanercept in 2009, elevated transaminase levels were detected on routine testing (alanine aminotransferase, 710IU/L; aspartate aminotransferase, 647IU/L). When the blood tests and viral serology were repeated, elevated liver enzyme levels were confirmed and HCV seroconversion was revealed. The patient was referred to the gastroenterology department, where complementary tests were ordered. The diagnosis was acute HCV infection (viral load>10 million copies/mL, genotype 1a). The patient reported not having received transfusions or undergone surgery recently. He denied parenteral drug use and high-risk sexual activities. The probable source of contact was judged to be his wife, who was found to harbor HCV. This chronic infection had not been diagnosed before her husband's acute infection led to testing.
Etanercept treatment was not interrupted. Given the patient's progressively increasing transaminase levels and viral load, pegylated interferon-alpha was prescribed in November 2009. Transaminase levels and viral load responded rapidly, but worsening psoriasis symptoms were an expected effect of the interferon treatment. A higher etanercept dose (100mg/wk) achieved partial response. The antiviral treatment was continued for 24 weeks, and sustained viral response was confirmed at the time of writing, The higher dose of etanercept was used throughout the duration of interferon treatment and in the month after antiviral therapy was stopped.
DiscussionTNF, a proinflammatory cytokine secreted by monocytes and macrophages, is implicated in the immune response to infections. Treatment with anti-TNF agents therefore increases the likelihood that latent bacterial (e.g., mycobacterial) or fungal infections will be reactivated. However, the effect of these agents on viral infections is less well understood.1–3
In hepatitis viral infection, elevated liver and serum levels of TNF and TNF receptors have been described.1,3,4 In HBV infection, TNF plays a key role in controlling viral replication.3 However, elevated levels of this cytokine are associated with greater hepatocellular damage in HCV infection.1 TNF also appears to be implicated in the pathogenesis of fibrosis of the liver, which leads to cirrhosis in 20% of HCV-infected patients over a period of 20 years.2 Anti-TNF agents might therefore even prove beneficial for some of these patients.1,2,4 In our patient, treatment with etanercept may have attenuated liver damage secondary to the primary acute HCV infection.
Regarding the possible adverse effect of anti-TNF agents on viral load or disease progression, relevant information can be inferred from reports of more than 80 cases involving chronically HCV-infected patients under such treatment (particularly with etanercept) for a variety of autoimmune diseases.4–9 The absence of significant changes in viral load and transaminase levels is a striking observation in these cases. Paradisi and coworkers5 recently reported the results of liver biopsy at baseline and after 12 months of etanercept treatment in 2 HCV-infected patients with psoriasis, observing no progression of liver damage. The use of anti-TNF agents in patients with chronic HCV infection therefore seems to be safe based on existing evidence.2–4 However, we were unable to find published cases like the one we report, in which acute HCV infection occurred during anti-TNF treatment. Our patient's course suggests that continuing etanercept in a primary infection like this one will not have an adverse effect on disease progression or the response to interferon, although further confirmation is required.
Elevated TNF levels potentially also interfere with the response to antiviral treatment in HCV.10,11 In a small double-blind, randomized placebo-controlled trial that assessed the possibility that etanercept might boost the effect of interferon–ribavirin treatment of chronic HCV infection, the authors reported that the negativization of viral load was significantly greater in the etanercept group (63%) than in the placebo group (32%).12 In the case we report, it is possible that in addition to a lack of an adverse effect of continued etanercept administration on the efficacy of the antiviral regimen, etanercept may even have assisted HCV-RNA negativization.
Finally, the fact that transmission in this case was by heterosexual contact suggests that even though clinical guidelines state that risk is low for this route,13 treatment with a biologic might increase the risk. Evidently, an exhaustive medical history should be taken for all patients on these drugs. Candidates for anti-TNF therapy are screened for contact with tuberculosis. It might also be useful to investigate the possibility of exposure to other diseases, especially through contact with relatives or other persons who live with the patient, so that we are not taken by surprise by infections that might compromise our patients’ safety during treatment. HBV vaccination is also necessary for patients with negative HBV serology in order to guard against primary infection during treatment with a biologic agent.
This is the first report of a case of primary HCV infection during a period of anti-TNF therapy. The course of disease in our patient suggests that it is safe to maintain etanercept treatment in cases of acute HCV infection. Just as etanercept does not have an adverse effect on the treatment of chronic infection, there seems to be no interference in acute infection either. This observation should be confirmed in larger series.
Conflicts of InterestDr M. Armengot-Carbó has an agreement with Pfizer to collaborate on another publication.
Dr M. Velasco and Dr E. Gimeno have participated in clinical trials, provided consulting services, received speaking fees, or accepted funding to attend conferences or training sessions from the following laboratories: Pfizer, Abbott, and Janssen-Cilag.
Dr R. Giner declares that she has no conflicts of interest.
Please cite this article as: Armengot-Carbó M, et al. Hepatitis C aguda en un paciente en tratamiento con etanercept. Actas Dermosifiliogr. 2013;104:239–41.