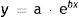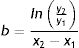Spain is in a situation of indefinite lockdown due to the ongoing coronavirus disease 2019 (COVID-19) pandemic. One of the consequences of this lockdown is delays in medical and surgical procedures for common diseases. The aim of this study was to model the impact on survival of tumor growth caused by such delays in patients with squamous cell carcinoma (SCC) and melanoma.
Material and methodsMulticenter, retrospective, observational cohort study. We constructed an exponential growth model for both SCC and melanoma to estimate tumor growth between patient-reported onset and surgical excision at different time points.
ResultsData from 200 patients with SCC of the head and neck and 1000 patients with cutaneous melanoma were included. An exponential growth curve was calculated for each tumor type and we estimated tumor size after 1, 2, and 3 months of potential surgical delay. The proportion of patients with T3 SCC (diameter > 4 cm or thickness > 6 mm) increased from 41.5% (83 patients) in the initial study group to an estimated 58.5%, 70.5%, and 72% after 1, 2, and 3 months of delay. Disease-specific survival at 2, 5, and 10 years in patients whose surgery was delayed by 3 months decreased by 6.2%, 8.2%, and 5.2%, respectively. The proportion of patients with ultrathick melanoma (> 6 mm) increased from 6.9% in the initial study group to 21.9%, 30.2%, and 30.2% at 1, 2, and 3 months. Five- and 10-year disease-specific survival both decreased by 14.4% in patients treated after a potential delay of 3 months.
ConclusionsIn the absence of adequate diagnosis and treatment of SCC and melanoma in the current lockdown situation in Spain, we can expect to see to a considerable increase in large and thick SCCs and melanomas. Efforts must be taken to encourage self-examination and facilitate access to dermatologists in order to prevent further delays.
La pandemia del coronavirus COVID-19 ha provocado un confinamiento indefinido. Una posible consecuencia de esta situación es un retraso en los procedimientos asistenciales de las patologías comunes. El objetivo de este estudio es estimar el hipotético impacto en la supervivencia que tendría el aumento del tamaño tanto para los carcinomas de células escamosas (CCE) como de los melanomas.
Material y métodoEstudio observacional retrospectivo de cohortes multicéntrico. Se desarrolló un modelo de crecimiento exponencial para cada tumor basado en el tiempo de evolución que refiere el paciente.
ResultadosSe incluyeron un total de 200 pacientes con CCEs localizados en la cabeza y el cuello y 1000 pacientes con melanoma cutáneo. Se calculó una curva de crecimiento exponencial para cada tumor y se estimó el tamaño del tumor tras 1, 2 y 3 mes tras el diagnóstico. En la muestra, los CCE mayores de 4 cm o >6 mm de grosor (definidos como T3) pasaron de 83 (41.5%) en el grupo de estudio real a una estimación de 58,5%, 70,5% y 72% tras 1, 2 y 3 meses de retraso quirúrgico estimado. Se estimó una disminución de la supervivencia específica de enfermedad (SEE) de un 6,2%, 8,2% y 5,2% a los 2, 5 y 10 años, respectivamente, tras tres meses de retraso. Para los melanomas, los melanomas ultragruesos (>6 mm) pasaron del 6,9% en el grupo de estudio al 21,9%, 30,2% y 30,2% tras 1, 2 y 3 meses de demora. La SEE a los 5 y 10 años del grupo de estudio descendió un 14,4% en ambos tiempos.
ConclusionesEn ausencia de un adecuado diagnóstico y tratamiento de los pacientes con CCE y melanoma en la actual situación de confinamiento en España, podemos llegar a asistir a un considerable aumento de los casos de CCE y melanomas gruesos y de gran tamaño. Se deben fomentar los esfuerzos para promocionar la autoexploración y facilitar el acceso a los dermatólogos para no aumentar la demora de estos pacientes.
The coronavirus disease 19 (COVID-19) pandemic, which started in Wuhan, China several months ago,1 has led to a large-scale lockdown in many countries across the world, including Spain. Uncertainty regarding the duration of the lockdown measures led us to consider how potential diagnostic and treatment delays due to the interruption of certain health procedures and services might affect the prognosis of patients with skin cancer. Diagnostic delays are known to be associated with larger tumor size in this setting.2 Tumor size at diagnosis also appears to be influenced by time to diagnosis and growth rate for both squamous cell carcinoma (SCC)3–5 and melanoma.6,7
The aim of this study was to estimate the extent to which surgical delays of 1, 2, and 3 months might affect survival and tumor size and/or thickness in SCC and melanoma, 2 of the most lethal skin cancers.8
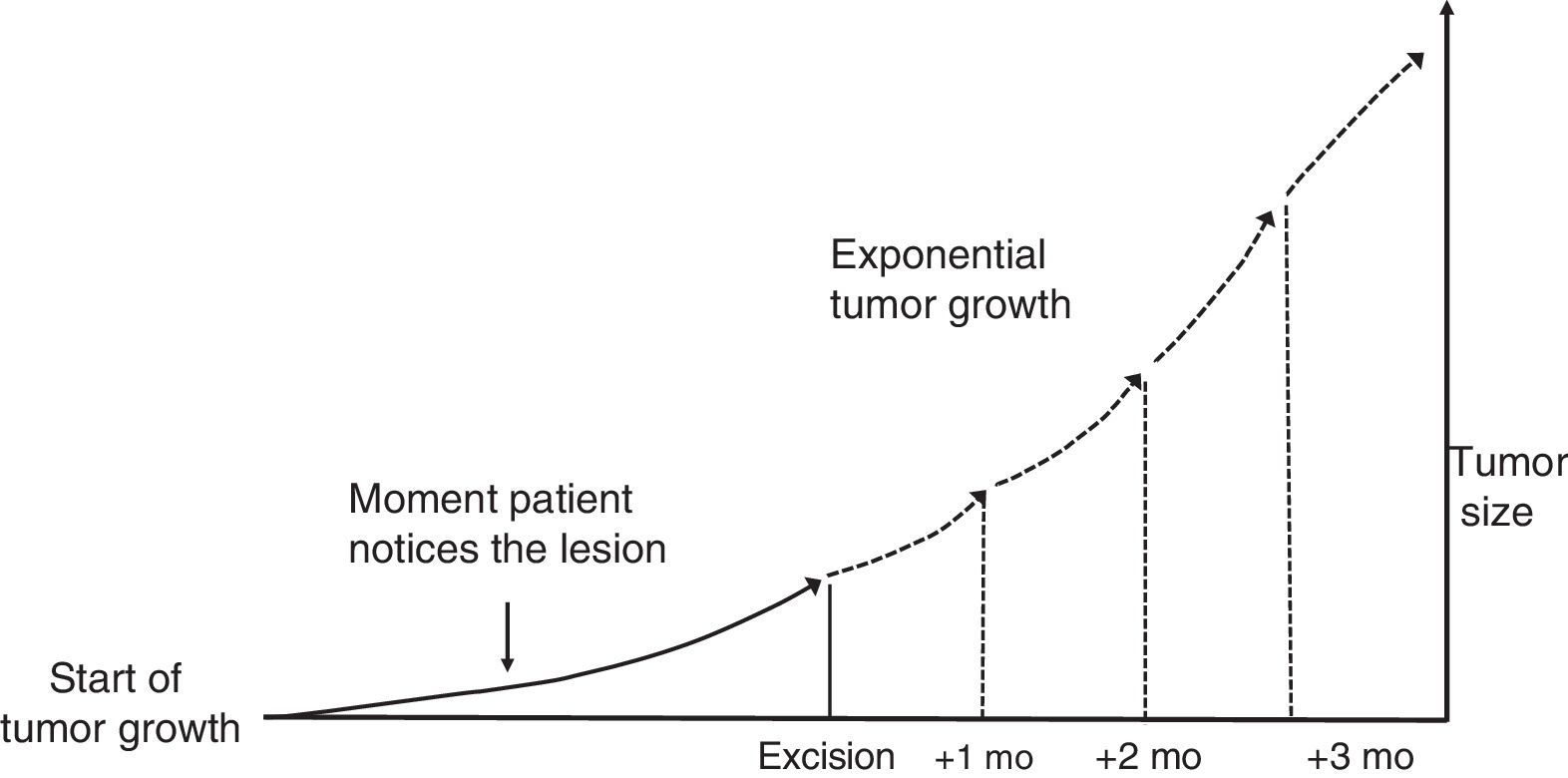
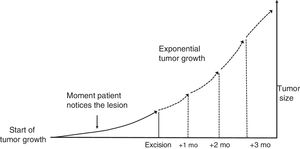
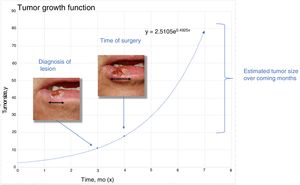
Material and MethodsTheoretical Framework and Case StudiesThe assumption on which this study is based is that tumors grow exponentially (Fig. 1).9 Although it could be argued that tumor growth follows a Gompertz, sigmoid-like, curve, we assumed that the models would be superimposable for relatively small tumors.10
The exponential function that links the dependent variable y (tumor size) to the independent variable x (time) is expressed as follows:
where a and b are both adjustable. It follows that at the beginning of tumor growth (x = 0) a is the initial size of the tumor, such thaty=a·e0, and b is the coefficient that determines its growth. The higher this second coefficient, the faster the tumor will grow.To estimate the exponential growth of a tumor, it is necessary to know y (size) and x (time) at 2 moments in time. In brief, y1 is the size of the tumor when it is first noticed by the patient and/or a relative, y2 is the size of the tumor at the time of surgery, x1 is the time when the tumor is first noticed, and x2 is the time of surgery. Asy1 (the size of the tumor when it is first noticed) is unknown, it was estimated for both SCCs and melanomas.
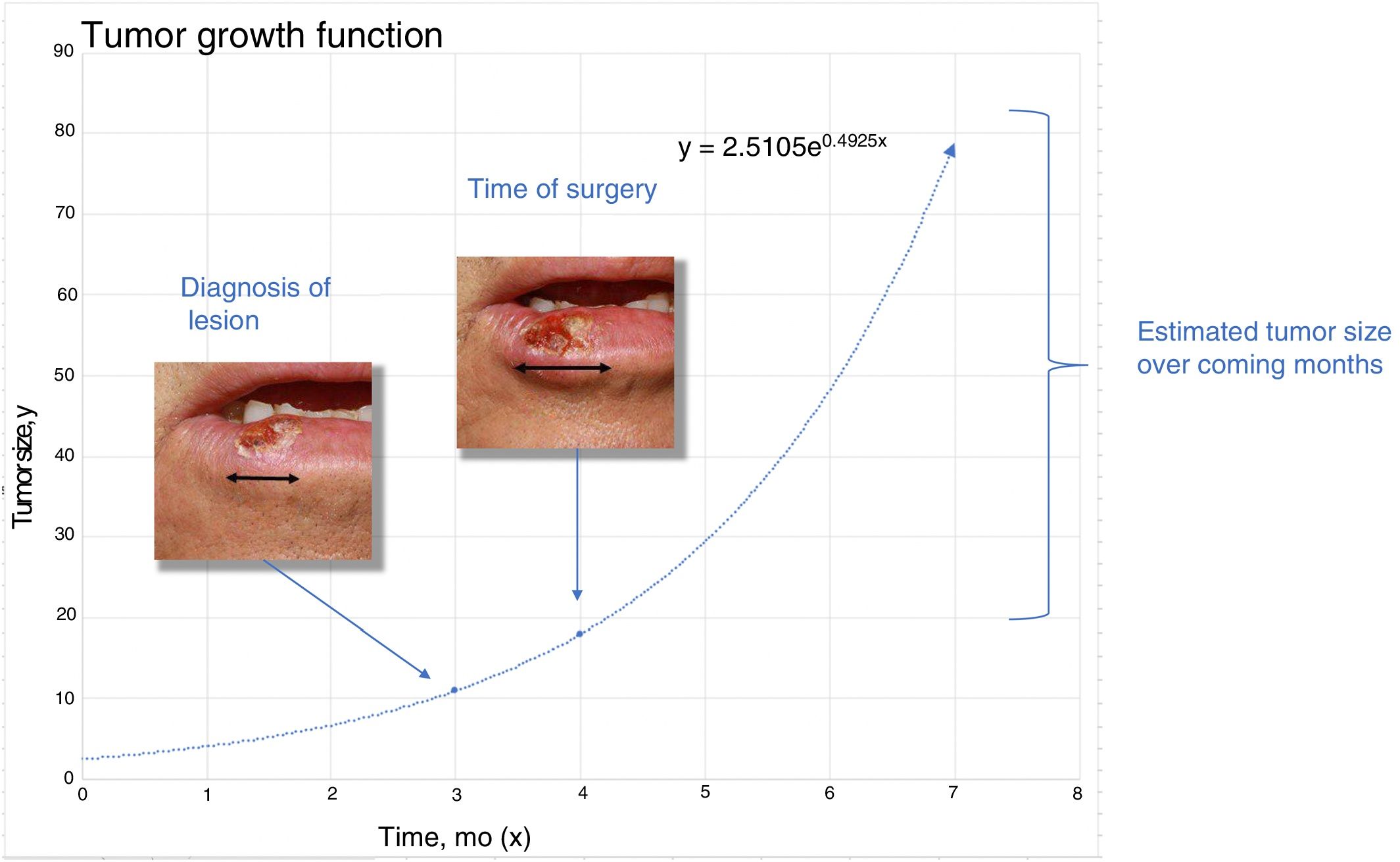
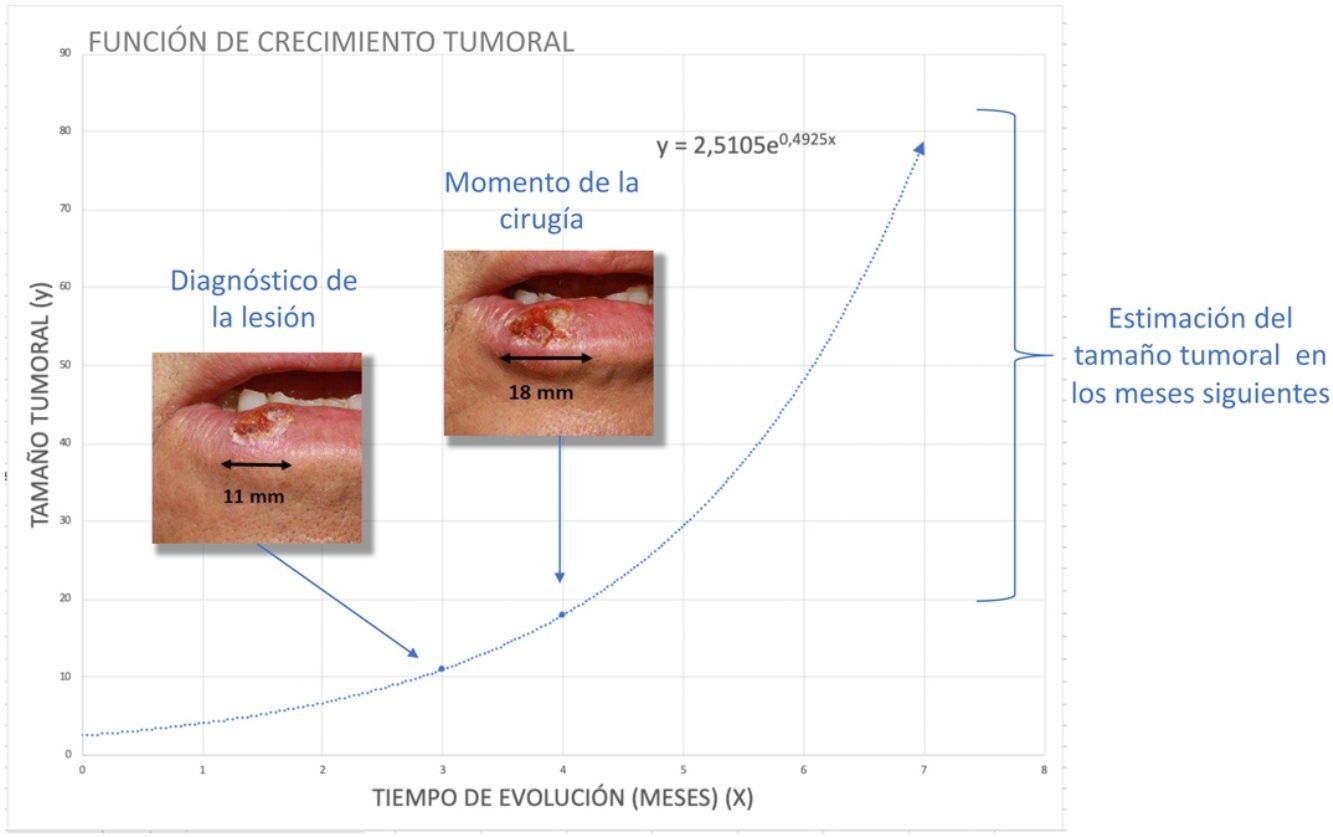
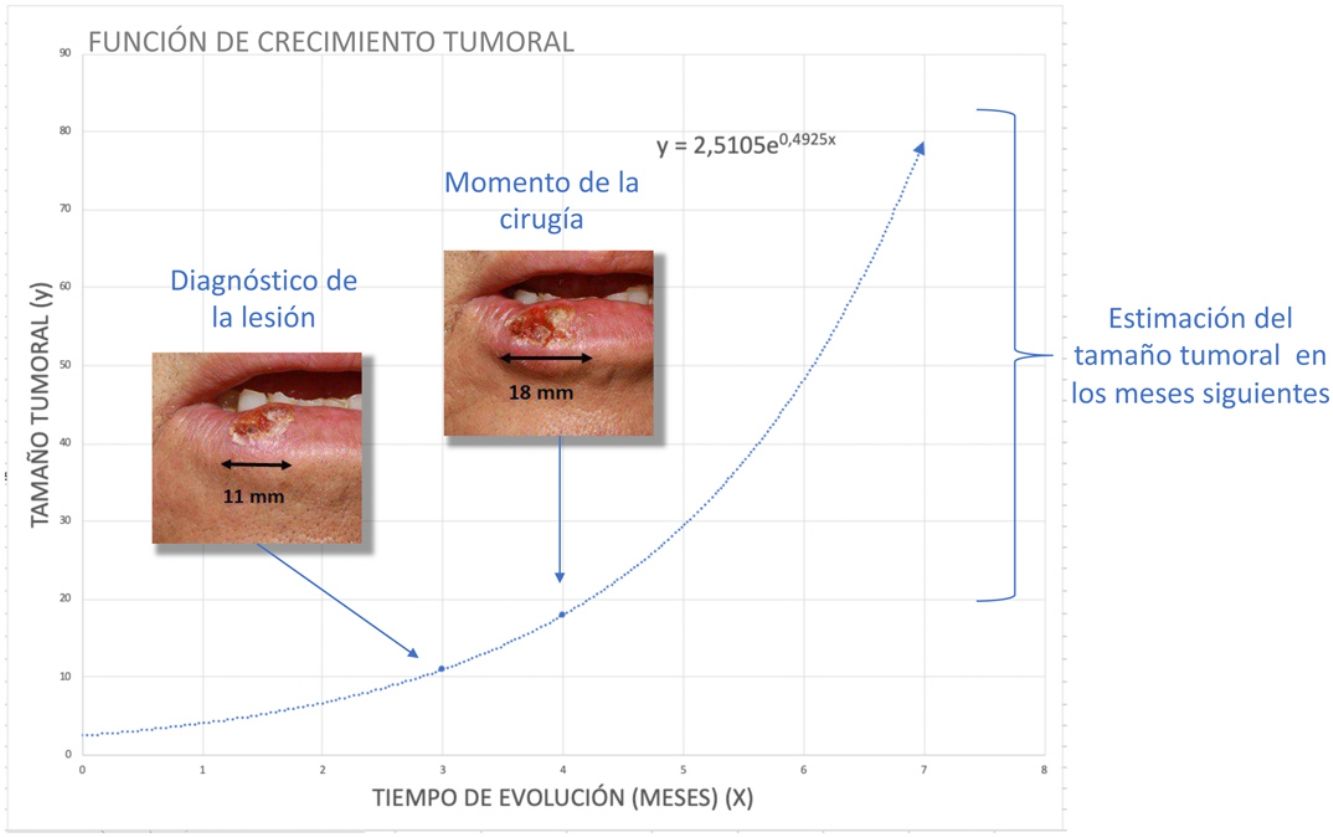
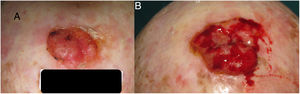
To estimatey1 for SCCs, we used data from a series of 6 cases that provided details of tumor size at 2 points in time (Fig. 2) (Appendix B Table 1 of the supplementary material). With this information, we calculated the exponential growth function for each of the 6 tumors (Fig. 3), where a was the diameter of the tumor at y1(when it was first noticed by the patient and/or a relative). The mean of the 6 measurements (a = 8.75 mm) was used as coefficient a to estimate the exponential growth of the SCCs in our sample. The same method was applied to estimate SCC thickness, which was known for 4 of the 6 tumors. Tumor thickness at diagnosis (y1) was assumed to be proportional to the diameter calculated for the same time point. The resulting thickness was 1.2 mm, which was used as coefficient a to calculate the exponential function for each of the SCCs in the study sample.
We were unable to perform similar measurements for melanomas, as we did not have details of mean Breslow thickness at 2 different time points. We therefore established a thickness of 0.2 mm for coefficient a based on the assumption that this would be the minimum vertical growth needed for the tumor to be noticed by the patient; x2 was defined as the time from when the patient noticed the tumor to the time of surgery.
Once we had estimated initial tumor size, we obtained coefficient b of the exponential function as follows:
With this information, we then estimated tumor size and thickness for SCCs and tumor thickness for melanomas after a hypothetical surgical delay of 1, 2, and 3 months.
We analyzed 200 SCCs and 1000 melanomas randomly selected from 2 databases.
The primary outcome measure was change in estimated tumor size and/or thickness at 1, 2, and 3 months. The results were categorized by size and thickness. SCCs were classified using a similar system to the SCC staging system of the American Joint Committee on Cancer (AJCC)11: T1 (diameter ≤ 2 cm), T2 (diameter 2−4 cm), and T3 (diameter > 4 cm or thickness > 6 mm). Melanomas were grouped into 6 Breslow thickness categories, similar to those proposed by the AJCC: < 0.8 mm, 0.8−1 mm, > 1−2 mm, > 2−4 mm, > 4−6 mm, and > 6 mm. The last 2 categories of thick tumors are not part of the AJCC staging system but were included as ultrathick melanomas were recently reported to have prognostic significance.12
We also performed a stratified analysis by sex and age (≤ 80 vs. > 80 years for SCC and ≤ 70 vs. > 70 years for melanoma).12
To analyze the impact of surgical delays on survival, we estimated disease-specific survival (DSS) for patients with SCC and melanoma overall and stratified by sex and age using survival curves from the Squamata database for SCCs (n = 434) (Figs. 1–5 of the supplementary material) and the melanoma database of the Instituto Valenciano de Oncología (n = 1637) (Figs. 6–10 of the supplementary material). The resulting survival rates (overall and by sex and age) were then used to estimate survival for patients with SCC and melanoma after a potential surgical delay of 1, 2, and 3 months. The Kaplan-Meier method was used to estimate overall and sex- and age-stratified DSS for the SCCs and melanomas analyzed.
The study was approved by the ethics committee of Hospital Universitario Reina Sofía Córdoba (reference 3958, file no. 280).
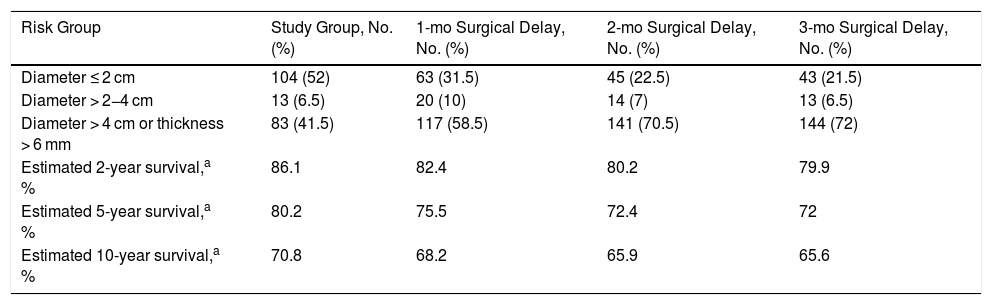
ResultsSquamous Cell CarcinomaOf the 200 SCCS with a known time from onset to surgery analyzed, 52% had a diameter of < 2 cm (T1). Just 13 (6.5%) had a diameter of 2−4 cm (T2) and 83 (41.5 %) had a diameter of > 4 cm or a thickness of > 6 mm (T3) (Table 1). The proportion of T3 tumors increased by 58.5% for a hypothetical surgical delay of 1 month, by 70.5% for a delay of 2 months, and by 72% for a delay of 3 months.
Tumor Size and Thickness at Diagnosis and After an Estimated Surgical Delay of 1, 2, and 3 Months Based on the Exponential Growth of 200 Squamous Cell Carcinomas of the Head and Neck from the Squamata Database.
| Risk Group | Study Group, No. (%) | 1-mo Surgical Delay, No. (%) | 2-mo Surgical Delay, No. (%) | 3-mo Surgical Delay, No. (%) |
|---|---|---|---|---|
| Diameter ≤ 2 cm | 104 (52) | 63 (31.5) | 45 (22.5) | 43 (21.5) |
| Diameter > 2−4 cm | 13 (6.5) | 20 (10) | 14 (7) | 13 (6.5) |
| Diameter > 4 cm or thickness > 6 mm | 83 (41.5) | 117 (58.5) | 141 (70.5) | 144 (72) |
| Estimated 2-year survival,a % | 86.1 | 82.4 | 80.2 | 79.9 |
| Estimated 5-year survival,a % | 80.2 | 75.5 | 72.4 | 72 |
| Estimated 10-year survival,a % | 70.8 | 68.2 | 65.9 | 65.6 |
Based on survival data for 434 patients from the Squamata database (Fig. 1 of the supplementary material).
Estimated DSS for the overall group was 86.1% at 2 years, 80.2% at 5 years, and 70.8% at 10 years. Survival rates decreased progressively over time to 79.9% for a delay of 1 month, 72% for a delay of 2 months, and 65.6% for a delay of 3 months.
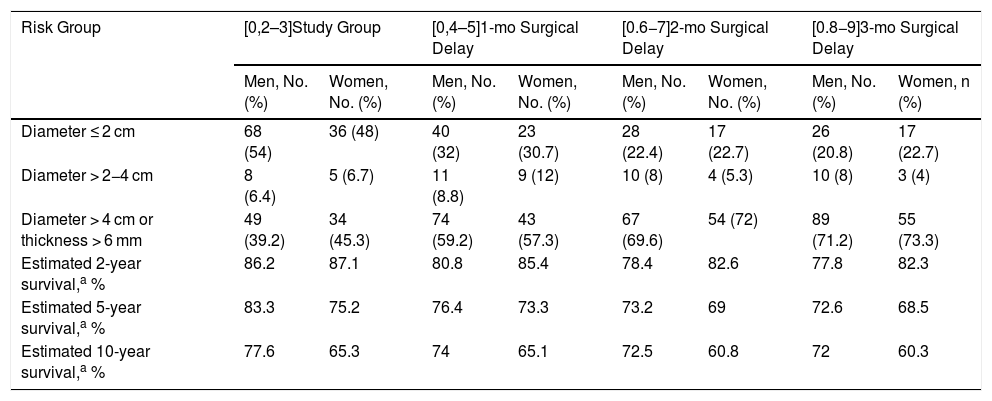
In the sex-stratified analysis, female sex was associated with worse 5-year DSS (75.2% vs. 83.3%) and 10-year DSS (65.3% vs. 77.6%). Again, survival rates decreased progressively over the 3 months. At the 3-month point, 5-year survival was 68.5% for women and 72.6% for men (Table 2).
Tumor Size and Thickness at Diagnosis and After an Estimated Surgical Delay of 1, 2, and 3 Months Based on the Exponential Growth of 200 Squamous Cell Carcinomas of the Head and Neck from the Squamata Database: Sex-Stratified Analysis.
| Risk Group | [0,2–3]Study Group | [0,4–5]1-mo Surgical Delay | [0.6−7]2-mo Surgical Delay | [0.8−9]3-mo Surgical Delay | ||||
|---|---|---|---|---|---|---|---|---|
| Men, No. (%) | Women, No. (%) | Men, No. (%) | Women, No. (%) | Men, No. (%) | Women, No. (%) | Men, No. (%) | Women, n (%) | |
| Diameter ≤ 2 cm | 68 (54) | 36 (48) | 40 (32) | 23 (30.7) | 28 (22.4) | 17 (22.7) | 26 (20.8) | 17 (22.7) |
| Diameter > 2−4 cm | 8 (6.4) | 5 (6.7) | 11 (8.8) | 9 (12) | 10 (8) | 4 (5.3) | 10 (8) | 3 (4) |
| Diameter > 4 cm or thickness > 6 mm | 49 (39.2) | 34 (45.3) | 74 (59.2) | 43 (57.3) | 67 (69.6) | 54 (72) | 89 (71.2) | 55 (73.3) |
| Estimated 2-year survival,a % | 86.2 | 87.1 | 80.8 | 85.4 | 78.4 | 82.6 | 77.8 | 82.3 |
| Estimated 5-year survival,a % | 83.3 | 75.2 | 76.4 | 73.3 | 73.2 | 69 | 72.6 | 68.5 |
| Estimated 10-year survival,a % | 77.6 | 65.3 | 74 | 65.1 | 72.5 | 60.8 | 72 | 60.3 |
Based on survival data for 434 patients from the Squamata database (Figs. 2 and 3 of the supplementary material).
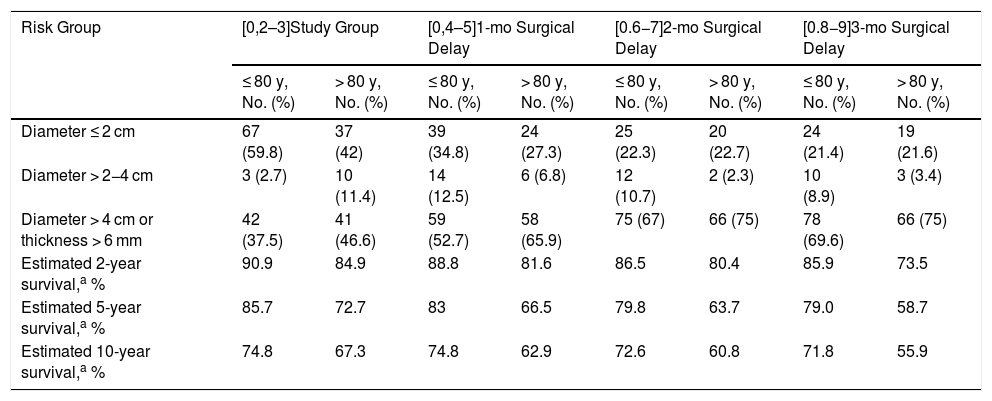
In the age-stratified analysis, DSS was worse in patients aged >80 years at all the time points analyzed, with the greatest difference observed at month 3 (5-year survival of 79% for patients aged > 80 years vs. 58.7% for those aged ≤ 80 years) (Table 3).
Tumor Size and Thickness at Diagnosis and After an Estimated Surgical Delay of 1, 2, and 3 Months Based on the Exponential Growth of 200 Squamous Cell Carcinomas of the Head and Neck from the Squamata Database: Age-Stratified Analysis.
| Risk Group | [0,2–3]Study Group | [0,4–5]1-mo Surgical Delay | [0.6−7]2-mo Surgical Delay | [0.8−9]3-mo Surgical Delay | ||||
|---|---|---|---|---|---|---|---|---|
| ≤ 80 y, No. (%) | > 80 y, No. (%) | ≤ 80 y, No. (%) | > 80 y, No. (%) | ≤ 80 y, No. (%) | > 80 y, No. (%) | ≤ 80 y, No. (%) | > 80 y, No. (%) | |
| Diameter ≤ 2 cm | 67 (59.8) | 37 (42) | 39 (34.8) | 24 (27.3) | 25 (22.3) | 20 (22.7) | 24 (21.4) | 19 (21.6) |
| Diameter > 2−4 cm | 3 (2.7) | 10 (11.4) | 14 (12.5) | 6 (6.8) | 12 (10.7) | 2 (2.3) | 10 (8.9) | 3 (3.4) |
| Diameter > 4 cm or thickness > 6 mm | 42 (37.5) | 41 (46.6) | 59 (52.7) | 58 (65.9) | 75 (67) | 66 (75) | 78 (69.6) | 66 (75) |
| Estimated 2-year survival,a % | 90.9 | 84.9 | 88.8 | 81.6 | 86.5 | 80.4 | 85.9 | 73.5 |
| Estimated 5-year survival,a % | 85.7 | 72.7 | 83 | 66.5 | 79.8 | 63.7 | 79.0 | 58.7 |
| Estimated 10-year survival,a % | 74.8 | 67.3 | 74.8 | 62.9 | 72.6 | 60.8 | 71.8 | 55.9 |
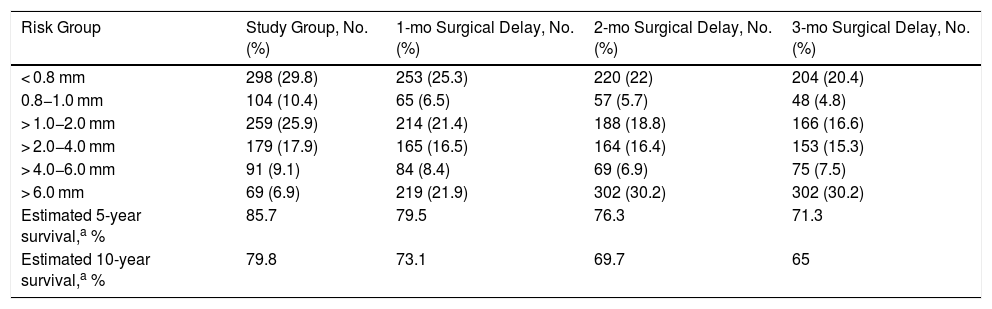
Most of the tumors from the melanoma database were thin; tumors measuring < 0.8 mm and 0.8−1 mm accounted for 29.8% and 10.4% of all tumors, respectively. Thick and ultrathick melanomas comprised the smallest group (9.1% for melanomas with a Breslow thickness of 4−6 mm and 6.9% for those with a Breslow thickness of > 6 mm) (Table 4). The greatest percentage increase over time was observed for tumors with a thickness of > 6 mm, which accounted for 21.9% of all tumors at 1 month, 30.2% at 2 months, and 30.2% at 3 months. Five- and 10-year DSS rates for the overall group were 85.7% and 79.4% respectively, and these decreased significantly over the 3 months analyzed, with the lowest rates observed at month 3 (71.3% for 5-year survival and 65% for 10-year survival) (Table 5).
Tumor thickness at Diagnosis and After an Estimated Surgical Delay of 1, 2, and 3 Months Based on the Exponential Growth of 1000 Melanomas from the Instituto Valenciano de Oncología Melanoma Database.
| Risk Group | Study Group, No. (%) | 1-mo Surgical Delay, No. (%) | 2-mo Surgical Delay, No. (%) | 3-mo Surgical Delay, No. (%) |
|---|---|---|---|---|
| < 0.8 mm | 298 (29.8) | 253 (25.3) | 220 (22) | 204 (20.4) |
| 0.8−1.0 mm | 104 (10.4) | 65 (6.5) | 57 (5.7) | 48 (4.8) |
| > 1.0−2.0 mm | 259 (25.9) | 214 (21.4) | 188 (18.8) | 166 (16.6) |
| > 2.0−4.0 mm | 179 (17.9) | 165 (16.5) | 164 (16.4) | 153 (15.3) |
| > 4.0−6.0 mm | 91 (9.1) | 84 (8.4) | 69 (6.9) | 75 (7.5) |
| > 6.0 mm | 69 (6.9) | 219 (21.9) | 302 (30.2) | 302 (30.2) |
| Estimated 5-year survival,a % | 85.7 | 79.5 | 76.3 | 71.3 |
| Estimated 10-year survival,a % | 79.8 | 73.1 | 69.7 | 65 |
Tumor thickness at Diagnosis and After an Estimated Surgical Delay of 1, 2, and 3 Months Based on the Exponential Growth of 1000 Melanomas from the Instituto Valenciano de Oncología Melanoma Database: Sex-Stratified Analysis.
| Risk Group | [0,2–3]Study Group | [0,4–5]1-mo Surgical Delay | [0.6−7]2-mo Surgical Delay | [0.8−9]3-mo Surgical Delay | ||||
|---|---|---|---|---|---|---|---|---|
| Men, No. (%) | Women, No. (%) | Men, No. (%) | Women, No. (%) | Men, No. (%) | Women, No. (%) | Men, No. (%) | Women, No. (%) | |
| < 0.8 mm | 122 (24.4) | 176 (35.2) | 106 (21.2) | 147 (29.4) | 93 (18.6) | 127 (25.4) | 84 (16.8) | 120 (24) |
| 0.8−1.0 mm | 39 (7.8) | 65 (13) | 24 (4.8) | 41 (8.2) | 21 (4.2) | 36 (7.2) | 19 (3.8) | 29 (5.8) |
| > 1.0−2.0 mm | 129 (25.8) | 130 (26) | 99 (19.8) | 115 (23) | 91 (18.2) | 97 (19.4) | 77 (15.4) | 89 (17.8) |
| > 2.0−4.0 mm | 109 (21.8) | 70 (14) | 81 (16.2) | 84 (16.8) | 74 (14.8) | 90 (18) | 74 (14.8) | 79 (15.8) |
| > 4.0−6.0 mm | 61 (12.2) | 30 (6) | 55 (11) | 29 (5.8) | 42 (8.4) | 27 (5.4) | 37 (7.4) | 38 (7.6) |
| > 6.0 mm | 40 (8) | 29 (5.8) | 135 (27) | 84 (16.8) | 179 (35.8) | 123 (24.6) | 209 (41.8) | 145 (29) |
| Estimated 5-year survival,a % | 82.8 | 89.2 | 75.7 | 83.5 | 73 | 79.8 | 70.7 | 77.5 |
| Estimated 10-year survival,a % | 76.1 | 83.5 | 68.8 | 77.2 | 66 | 73.2 | 63.6 | 70.9 |
In the sex-stratified analysis, ultrathick melanomas (> 6 mm) were somewhat more common in men (8% vs. 5.8% for women) both initially and at the 3 time points analyzed. The increase in the proportion of these tumors was significant from 1 month onwards (27% for men vs. 16.8% for women) and peaked at month 3 (41.8% vs. 29%).
Five-year DSS for the overall group was better in women (89.2% vs 82.8%), but it decreased in both groups over the following months, reaching its lowest point at month 3 (77.5% for women vs. 70.7% for men).
In the age-stratified analysis, thick melanomas (> 4−6 mm) were more common in patients aged > 70 years (16.8% vs 7.2% in patients aged ≤ 70 years), while ultrathick melanomas were present to a similar degree in both age categories (5.8% vs. 5%). A significant increase in the proportion of ultrathick tumors was observed for each of the 3 time points analyzed, and was particularly high in both age groups at month 3 (52% for patients aged > 70 years vs. 31.3% for those aged ≤ 70 years) (Table 6).
Tumor thickness at Diagnosis and After an Estimated Surgical Delay of 1, 2, and 3 Months Based on the Exponential Growth of 1000 Melanomas from the Instituto Valenciano de Oncología Melanoma Database: Age-Stratified Analysis.
| Risk Group | [0,2–3]Study Group | [0,4–5]1-mo Surgical Delay | [0.6−7]2-mo Surgical Delay | [0.8−9]3-mo Surgical Delay | ||||
|---|---|---|---|---|---|---|---|---|
| ≤ 70 y, No. (%) | > 70 y, No. (%) | ≤ 70 y, No. (%) | > 70 y, No. (%) | ≤ 70 y, No. (%) | > 70 y, No. (%) | ≤ 70 y, No. (%) | > 70 y, No. (%) | |
| < 0.8 mm | 265 (33) | 33 (16.8) | 222 (27.6) | 31 (15.8) | 193 (24) | 27 (13.8) | 179 (22.3) | 25 (12.8) |
| 0 8−1.0 mm | 93 (11.6) | 11 (5.6) | 59 (7.3) | 6 (3.1) | 49 (6.1) | 8 (4.1) | 41 (5.1) | 7 (3.6) |
| > 1.0−2.0 mm | 218 (27.1) | 41 (20.9) | 185 (23) | 29 (14.8) | 166 (20.6) | 22 (11.2) | 146 (18.2) | 20 (10.2) |
| > 2.0−4.0 mm | 130 (16.2) | 49 (25) | 132 (16.4) | 33 (16.8) | 133 (16.5) | 31 (15.8) | 126 (15.7) | 27 (13.8) |
| > 4.0−6.0 mm | 58 (7.2) | 33 (16.8) | 57 (7.1) | 27 (13.8) | 53 (6.6) | 16 (8.2) | 60 (7.5) | 15 (7.7) |
| > 6.0 mm | 40 (5) | 29 (5.8) | 149 (18.5) | 70 (35.7) | 210 (26.1) | 92 (46.9) | 252 (31.3) | 102 (52) |
| Estimated 5-year survival,a % | 88.4 | 86.3 | 81.7 | 81.7 | 78.2 | 78.2 | 75.7 | 75.7 |
| Estimated 10-year survival,a % | 83.5 | 75.6 | 77.1 | 68.3 | 73.9 | 64.5 | 71.6 | 61.9 |
Patients in the over-70 category experienced the greatest change in survival rates from baseline to month 3 (86.3% to 75.6% for 5-year survival and 75.7% to 61.9% for 10-year survival).
DiscussionThe main finding of this study is that delaying the surgical excision of SCC or melanoma by 1 month or longer increases the proportion of large or thick tumors and results in worse survival.
People in Spain and many other countries around the world have been confined to their homes for several weeks now due to the COVID-19 pandemic. Significant time and resources are being invested to learn more about this disease and about how to manage it both now and in the future. There is little discussion, however, about how the vast diversion of healthcare resources towards the COVID-19 outbreak might affect other diseases, such as cancer. In recent weeks, numerous governments have ruled that doctors should not deal with deferrable medical procedures. Cancer surgery, however, cannot be deferred and should continue during the pandemic. Nonetheless, there are signs that the provision of care to patients with severe illness is declining at an alarming rate. One oral medicine unit in Italy, for example, reported that it had diagnosed practically no cases of oral squamous cell carcinoma during the lockdown period,13 while an Austrian study reported a significant decline in hospital admissions for acute coronary syndrome in the first days of lockdown, which could result in an increase in infarct-related morbidity and mortality in the coming weeks.14 Similar concerns have been reported for prostate cancer.15
In the case of skin cancer, many patients feel confident that they can postpone seeking care for what is actually a malignant lesion,2 and in the current scenario, in many cases this behavior may be compounded by fear of becoming infected or infecting their family if they do seek care.
The COVID-19 pandemic has brought unprecedented times. While a patient with primary skin cancer should be offered surgery without delay as the first line of action, in light of the current shortages of equipment, healthcare personnel, and ICU beds,16 other options such as radiation therapy or intralesional methotrexate (for SCCs) could be contemplated in certain cases, such as slow-growing SCCs or thin tumors.17
Our study has some limitations. First, our findings are based on a mathematical estimation of a growth curve built using data from a small number of patients and assuming a scenario in which the entire population is affected by surgical delays. This may, however, not actually be the case, as some patients have undergone surgery during lockdown or continued to see a doctor or use teledermatology services. Second, the impact on melanoma thickness may be overestimated, as many tumors are thicker than the estimated thickness of 0.2 mm when they are first noticed by a patient. Third, we did not use the same stages as those proposed by the AJCC, as we were unable to determine which patients would develop ulceration, or, in the case of SCC, which patients would experience progression to perineural or bone invasion. Finally, the survival curves used to estimate survival were not adjusted for other variables.
Our study also has strengths. Although our sample was small, the distribution of melanoma thicknesses is almost identical to that in the Spanish Melanoma Registry.18 The proportion of thicker tumors in our sample of SCCs is higher than that documented in large series19 (probably because we included tumors with bone involvement or other poor prognostic factors), and this would have resulted in worse survival curves.
ConclusionsWe have shown that a failure to prioritize diagnosis and treatment of SCC and melanoma in the current lockdown situation in Spain could lead to a considerable increase in more advanced tumors and healthcare costs.9
Approximately 300 melanomas and 1500 SCCs are diagnosed in Spain every month.10 The potential impact of diagnostic and treatment delays in countries with similar mobility restrictions to Spain but even higher incidence rates is enormous.
To address this situation, prompt efforts are needed to promote self-examination and facilitate access to dermatologists (via teledermatology, for example).
Conflicts of interestThe authors declare that they have no conflicts of interest.
FundingThis study was partially funded by a grant from the Fundación Piel Sana (Healthy Skin Foundation) of the Spanish Academy of Dermatology and Venereology (AEDV). The research work of J.C. is partially funded by Instituto de Salud Carlos III (project PI18/00587), which is confunded by the European Regional Development Fund.
We would like to thank Inmaculada Morales Villagrás and Mónica Moya Pareja for their mathematical advice and Miguel Ángel Descalzo-Gallego for his statistical advice.
Please cite this article as: Tejera-Vaquerizo A, Cañueto J, Toll A, Santos-Juanes J, Jaka A, Ferrandiz C, et al. Estimación del efecto en el tamaño y la supervivencia de los tumores cutáneos debido al confinamiento por COVID-19: modelo basado en un crecimiento exponencial. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.05.001