Cutaneous squamous cell carcinoma (cSCC) is the second leading cause of skin cancer mortality in Europe. Few studies have analyzed the different pathways of this tumor progression in its natural history. The main objective of this study was to analyze the different metastatic and progression pathways and their temporal occurrence in the evolution of cSCC.
Material and methodWe conducted a multicenter, retrospective, and observational study of consecutive high-risk sSCCs included in the SQUAMATA project.
ResultsA total of 222 out of the 1346 patients included relapsed. The most frequent route of progression was the lymphatic one (62.6%). A total of 20.2% of the cases with lymphatic progression developed distant metastases. Only 1 case (3.1%) of distant metastasis followed local recurrence without previous lymphatic metastasis. The median time to disease-related mortality was longer in patients who developed systemic metastases than in those who died of locoregional progression.
ConclusionsThe mortality of patients with cSCC is mostly due to the regional progression of their lymphatic metastases. The appearance of distant metastases is practically always (96.9%) associated with previous lymphatic metastatic progression. Therefore, in the future, new studies will be needed to assess the regional management of cSCC in both surgical and adjuvant therapies.
El carcinoma cutáneo de células escamosas (CEC) es la segunda causa de fallecimientos por cáncer de piel en Europa. Existen pocos estudios que hayan analizado las distintas vías de progresión de este tumor en su historia natural. El objetivo principal del presente estudio ha sido analizar las diferentes vías metastásicas, así como de progresión, y su aparición temporal en la evolución del CEC.
Material y métodoEstudio observacional retrospectivo multicéntrico de los CEC consecutivos de alto riesgo englobados en el proyecto SQUAMATA.
ResultadosDe los 1.346 pacientes incluidos, tuvieron recaída 222 pacientes. La vía de progresión más frecuente fue la vía linfática (62,6%). El 20,2% de los casos con progresión linfática desarrollaron metástasis a distancia. Un solo caso (3,1%) de metástasis a distancia fue tras recidiva local sin metástasis linfáticas previas. La mediana del tiempo hasta el exitus por la enfermedad fue mayor en los pacientes que desarrollaron metástasis sistémicas que en aquellos fallecidos por progresión locorregional.
ConclusionesEl fallecimiento de los pacientes con CEC es mayoritariamente por progresión regional de sus metástasis linfáticas. La aparición de metástasis a distancia se asocia prácticamente siempre (96,9%) a la progresión metastásica linfática previa. En el futuro, por tanto, se hacen necesarios nuevos estudios que valoren el manejo regional del CEC tanto en su manejo quirúrgico como adyuvante.
Cutaneous squamous cell carcinoma (cSCC) is the second most frequent malignant cutaneous neoplasm in Spain.1 cSCC is also the second leading cause of skin cancer mortality after melanoma.1 This tumor has low metastatic potential. cSCC preferentially metastasizes via the lymphatic route in 3% up to 5% of cases, depending on the location, size, depth of invasion, perineural invasion, or bone involvement.2
On the other hand, selective sentinel lymph node biopsy (SLNB) is a surgical technique widely developed in other types of tumors, such as melanoma, based on its propensity for regional lymphatic metastases, without an impact on the survival of these patients.3 In the case of cSCC, the utility of SLNB is unknown to this date, with a positivity rate of 8% up to 10%.4
Very few studies have been published on the natural history in terms of cSCC progression,5 or on the clinical course of various relapse forms in this tumor progression.
Understanding and analyzing how cSCC progresses would help design diagnostic, therapeutic, and follow-up strategies for these patients.
The primary endpoint of this study is to analyze the different pathways of cSCC progression (local recurrence, satellitosis, regional lymphatic recurrence, or distant metastasis), along with the time course of these progressions. Secondary endpoints include analyzing the relationship of different clinical and pathological variables with the different forms of progression.
Material and methodsParticipants and study designWe conducted a retrospective, multicenter, and observational study, including patients from 8 reference hospitals participating in the SQUAMATA project. This project focuses on studying prognostic factors of cSCC and has been previously described.6 Participant hospitals are Hospital Universitario de Salamanca, Salamanca, Spain, Instituto Valenciano de Oncología, Valencia, Spain, Hospital Germans Trias i Pujol, Badalona, Spain, Hospital Clínic, Barcelona, Spain, Hospital Universitari Vall d’Hebron, Barcelona, Spain, Hospital Universitario Central de Asturias, Oviedo, Spain; Hospital San Cecilio, Granada, Spain, and University Hospital Città della Salute e della Scienza di Torino, Turin, Italy.
Patients diagnosed with high-risk cSCC from January 1st, 2000 through December 31st, 2020, were included. Tumors with a diameter ≥ 2cm, tumor thickness ≥ 6mm, presence of perineural or lymphovascular invasion, poor histological differentiation, invasion beyond subcutaneous fat, location in the ear or lip, and patient immunosuppression (solid organ transplantation, chronic lymphocytic leukemia, chronic immunosuppressive treatment, or chronic kidney disease) were considered high-risk cSCC.
In cases of multiple cSCC, only the tumor with the highest-risk characteristics was included in the database.
The study was approved by Hospital Universitario Reina Sofía research and ethics committee in Córdoba, Spain (registration No. 3958).
The follow-up of the different centers is similar and is fundamentally based on NCCN guidelines,7 with patient follow-up every 3 to 6 months within the first 2 years and then every 6-12 months from the 3rd year onwards, becoming annual from the 5th year. In the case of regional disease, controls are every 2-3 months within the 1st year, every 4-6 months up to year 3, and every 6-12 months from year 4. The imaging modalities used in the follow-ups are ultrasound, computed tomography (CT), and, in cases in which local spread of tissues needed to be assessed, magnetic resonance imaging (MRI) was used.
The first progression pathway was described for the purpose of the study. Based on this progression, 4 groups were defined: local recurrence (LR), regional lymphatic recurrence (L), satellitosis/transit metastasis (S), and distant metastasis (M).
The involvement of these 4 progression pathways can lead to various dissemination patterns, which is similar to those classically described for melanoma.8,9
For patients who ended up dying of this disease, 7 patterns or routes of dissemination were described: LR-d pattern, development of LR and the dead (d); LR-M-d pattern, development of LR and then M and d; LR-L-d pattern, development of LR, and then L, and eventually d; LR-L-M-d pattern, development of LR and then L, M, and eventually d; LR-d pattern, development of L and then d; L-M-d pattern, development of L, then M, and eventually d; and finally, S-d pattern, with the development of S and eventually d.
The chi-square test was used to compare differences between different metastatic pathways (LR, L, S, and M) and several categorical clinical and pathological variables (gender, immunosuppression, location on head/neck, trunk/extremities, and hands/feet, diameter ≤ 20mm vs >20mm, tumor thickness ≤ 6 vs >6mm, perineural invasion, lymphovascular invasion, histological grading, American Joint Committee on Cancer (AJCC) classification,10 and Brigham and Women's Hospital (BWH) classification.11 Age was evaluated using the Kruskal-Wallis test. Significant tests were evaluated with post hoc analyses, and with adjusted p-values using the Holm method. Kaplan-Meier (KM) curves and the log-rank test were used to compare the time elapsed until different forms of progression were reported. Similarly, KM curves were compared for regional lymphatic metastases as first recurrence or after local recurrence, as well as the same regional lymphatic metastases depending on whether they progressed regionally to systemic metastases or whether the patient remained alive. Finally, the time until progression was assessed based on the type of local, regional, or distant recurrence. P values <0.05 were considered statistically significant. IBM SPSS Statistics software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) was used for statistical analysis.
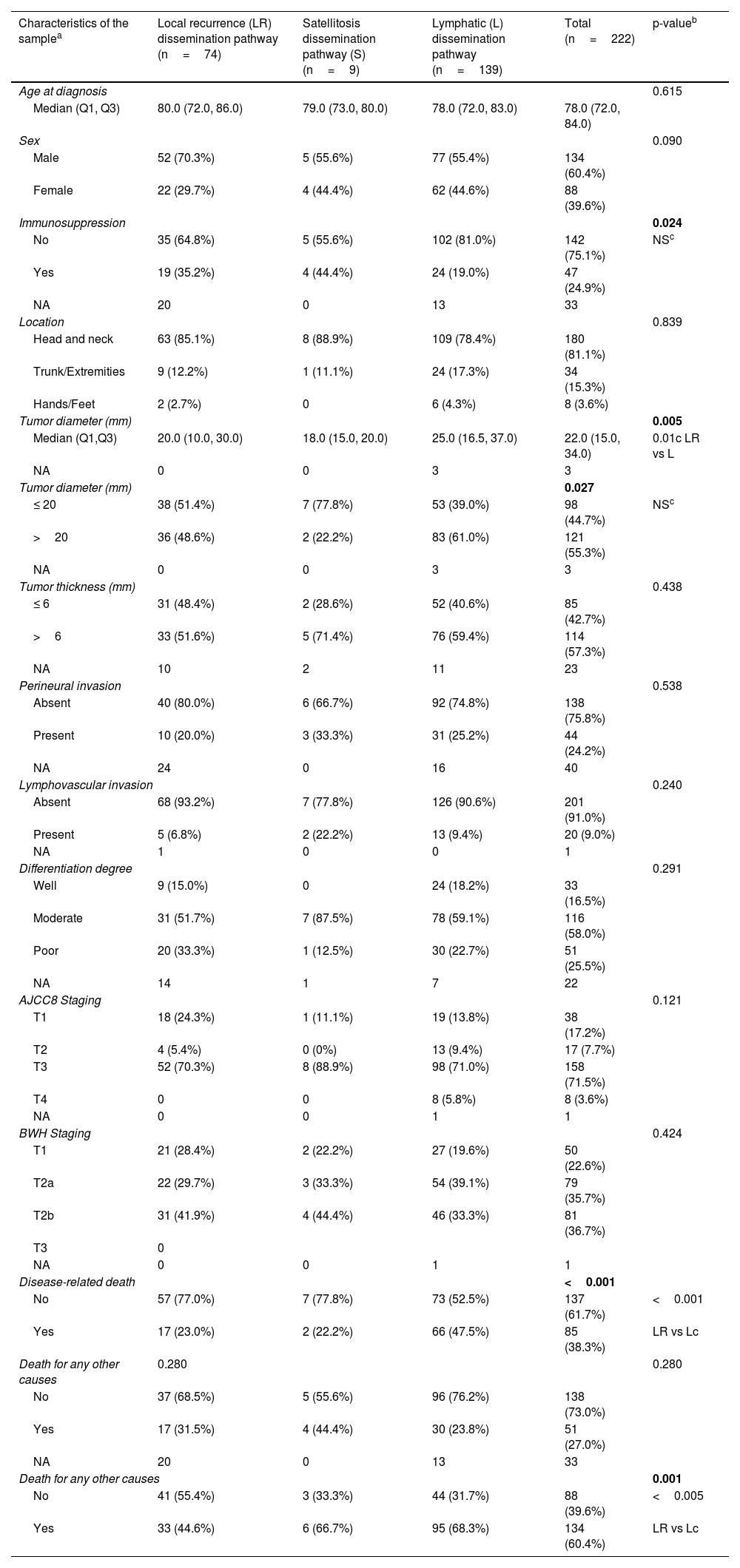
ResultsA total of 1346 patients with primary high-risk cSCC were included, 16.5% of whom (n=222) experienced some form of recurrence during follow-up. The median follow-up was 33 months. Table 1 shows the clinical and pathological characteristics of the population based on the different progression pathways. Statistically significant differences were found only in terms of immunosuppression status, tumor diameter, and the probability of mortality both from the neoplasm and from any other causes. The most common dissemination pathway in both immunocompetent and immunocompromised patients was lymphatic spread (71.8% and 51%, respectively), while local recurrences and dissemination via satellitosis were relatively more frequent in immunocompromised patients (40% vs 24.6% in immunocompetent). Lymphatic recurrences were somewhat more frequent in immunocompetent patients (71.8% vs 51.1%). Tumors that spread via lymphatic system were, on average, 5mm larger in diameter than those that recurred locally (25mm vs 20mm, p=0.01). Regarding tumors that spread via satellitosis as the primary dissemination pathway, we observed that most were tumors ≤ 20mm (77.8%), unlike those that recurred lymphatically, which were mostly >2cm (61.0%).
Clinical and pathological characteristics of high-risk skin carcinomas based on various dissemination pathways (n=222).
| Characteristics of the samplea | Local recurrence (LR) dissemination pathway (n=74) | Satellitosis dissemination pathway (S) (n=9) | Lymphatic (L) dissemination pathway (n=139) | Total (n=222) | p-valueb |
|---|---|---|---|---|---|
| Age at diagnosis | 0.615 | ||||
| Median (Q1, Q3) | 80.0 (72.0, 86.0) | 79.0 (73.0, 80.0) | 78.0 (72.0, 83.0) | 78.0 (72.0, 84.0) | |
| Sex | 0.090 | ||||
| Male | 52 (70.3%) | 5 (55.6%) | 77 (55.4%) | 134 (60.4%) | |
| Female | 22 (29.7%) | 4 (44.4%) | 62 (44.6%) | 88 (39.6%) | |
| Immunosuppression | 0.024 | ||||
| No | 35 (64.8%) | 5 (55.6%) | 102 (81.0%) | 142 (75.1%) | NSc |
| Yes | 19 (35.2%) | 4 (44.4%) | 24 (19.0%) | 47 (24.9%) | |
| NA | 20 | 0 | 13 | 33 | |
| Location | 0.839 | ||||
| Head and neck | 63 (85.1%) | 8 (88.9%) | 109 (78.4%) | 180 (81.1%) | |
| Trunk/Extremities | 9 (12.2%) | 1 (11.1%) | 24 (17.3%) | 34 (15.3%) | |
| Hands/Feet | 2 (2.7%) | 0 | 6 (4.3%) | 8 (3.6%) | |
| Tumor diameter (mm) | 0.005 | ||||
| Median (Q1,Q3) | 20.0 (10.0, 30.0) | 18.0 (15.0, 20.0) | 25.0 (16.5, 37.0) | 22.0 (15.0, 34.0) | 0.01c LR vs L |
| NA | 0 | 0 | 3 | 3 | |
| Tumor diameter (mm) | 0.027 | ||||
| ≤ 20 | 38 (51.4%) | 7 (77.8%) | 53 (39.0%) | 98 (44.7%) | NSc |
| >20 | 36 (48.6%) | 2 (22.2%) | 83 (61.0%) | 121 (55.3%) | |
| NA | 0 | 0 | 3 | 3 | |
| Tumor thickness (mm) | 0.438 | ||||
| ≤ 6 | 31 (48.4%) | 2 (28.6%) | 52 (40.6%) | 85 (42.7%) | |
| >6 | 33 (51.6%) | 5 (71.4%) | 76 (59.4%) | 114 (57.3%) | |
| NA | 10 | 2 | 11 | 23 | |
| Perineural invasion | 0.538 | ||||
| Absent | 40 (80.0%) | 6 (66.7%) | 92 (74.8%) | 138 (75.8%) | |
| Present | 10 (20.0%) | 3 (33.3%) | 31 (25.2%) | 44 (24.2%) | |
| NA | 24 | 0 | 16 | 40 | |
| Lymphovascular invasion | 0.240 | ||||
| Absent | 68 (93.2%) | 7 (77.8%) | 126 (90.6%) | 201 (91.0%) | |
| Present | 5 (6.8%) | 2 (22.2%) | 13 (9.4%) | 20 (9.0%) | |
| NA | 1 | 0 | 0 | 1 | |
| Differentiation degree | 0.291 | ||||
| Well | 9 (15.0%) | 0 | 24 (18.2%) | 33 (16.5%) | |
| Moderate | 31 (51.7%) | 7 (87.5%) | 78 (59.1%) | 116 (58.0%) | |
| Poor | 20 (33.3%) | 1 (12.5%) | 30 (22.7%) | 51 (25.5%) | |
| NA | 14 | 1 | 7 | 22 | |
| AJCC8 Staging | 0.121 | ||||
| T1 | 18 (24.3%) | 1 (11.1%) | 19 (13.8%) | 38 (17.2%) | |
| T2 | 4 (5.4%) | 0 (0%) | 13 (9.4%) | 17 (7.7%) | |
| T3 | 52 (70.3%) | 8 (88.9%) | 98 (71.0%) | 158 (71.5%) | |
| T4 | 0 | 0 | 8 (5.8%) | 8 (3.6%) | |
| NA | 0 | 0 | 1 | 1 | |
| BWH Staging | 0.424 | ||||
| T1 | 21 (28.4%) | 2 (22.2%) | 27 (19.6%) | 50 (22.6%) | |
| T2a | 22 (29.7%) | 3 (33.3%) | 54 (39.1%) | 79 (35.7%) | |
| T2b | 31 (41.9%) | 4 (44.4%) | 46 (33.3%) | 81 (36.7%) | |
| T3 | 0 | ||||
| NA | 0 | 0 | 1 | 1 | |
| Disease-related death | <0.001 | ||||
| No | 57 (77.0%) | 7 (77.8%) | 73 (52.5%) | 137 (61.7%) | <0.001 |
| Yes | 17 (23.0%) | 2 (22.2%) | 66 (47.5%) | 85 (38.3%) | LR vs Lc |
| Death for any other causes | 0.280 | 0.280 | |||
| No | 37 (68.5%) | 5 (55.6%) | 96 (76.2%) | 138 (73.0%) | |
| Yes | 17 (31.5%) | 4 (44.4%) | 30 (23.8%) | 51 (27.0%) | |
| NA | 20 | 0 | 13 | 33 | |
| Death for any other causes | 0.001 | ||||
| No | 41 (55.4%) | 3 (33.3%) | 44 (31.7%) | 88 (39.6%) | <0.005 |
| Yes | 33 (44.6%) | 6 (66.7%) | 95 (68.3%) | 134 (60.4%) | LR vs Lc |
AJCC-8, American Joint Committee on Cancer VIII edition; BWH, Brigham and Women's Hospital classification; L, lymphatic; LR, local recurrence; NA, not available; NS, nonstatistically significant; Q1, Q2, 1st and 3rd quartiles; S, satellitosis.
Regarding staging, we should mention that, all T4 tumors according to AJCC and all T3 tumors according to BWH preferentially spread to lymph nodes (100%). On the other hand, low-risk T1 tumors that progressed did so almost equally as local recurrences or lymph node metastases (table 1).
Regarding mortality according to pathways, tumors that presented with a local recurrence had a lower specific mortality rate than those presenting in lymph nodes (23% vs 47.5%, p <0.001), and a lower overall mortality rate too (44.6% vs 68.3%, p<0.005) (table 1).
Of note that neither age, sex, location, differentiation grade, tumor thickness, perineural or lymphovascular invasion, nor staging were significantly differentially distributed among the different dissemination pathways.
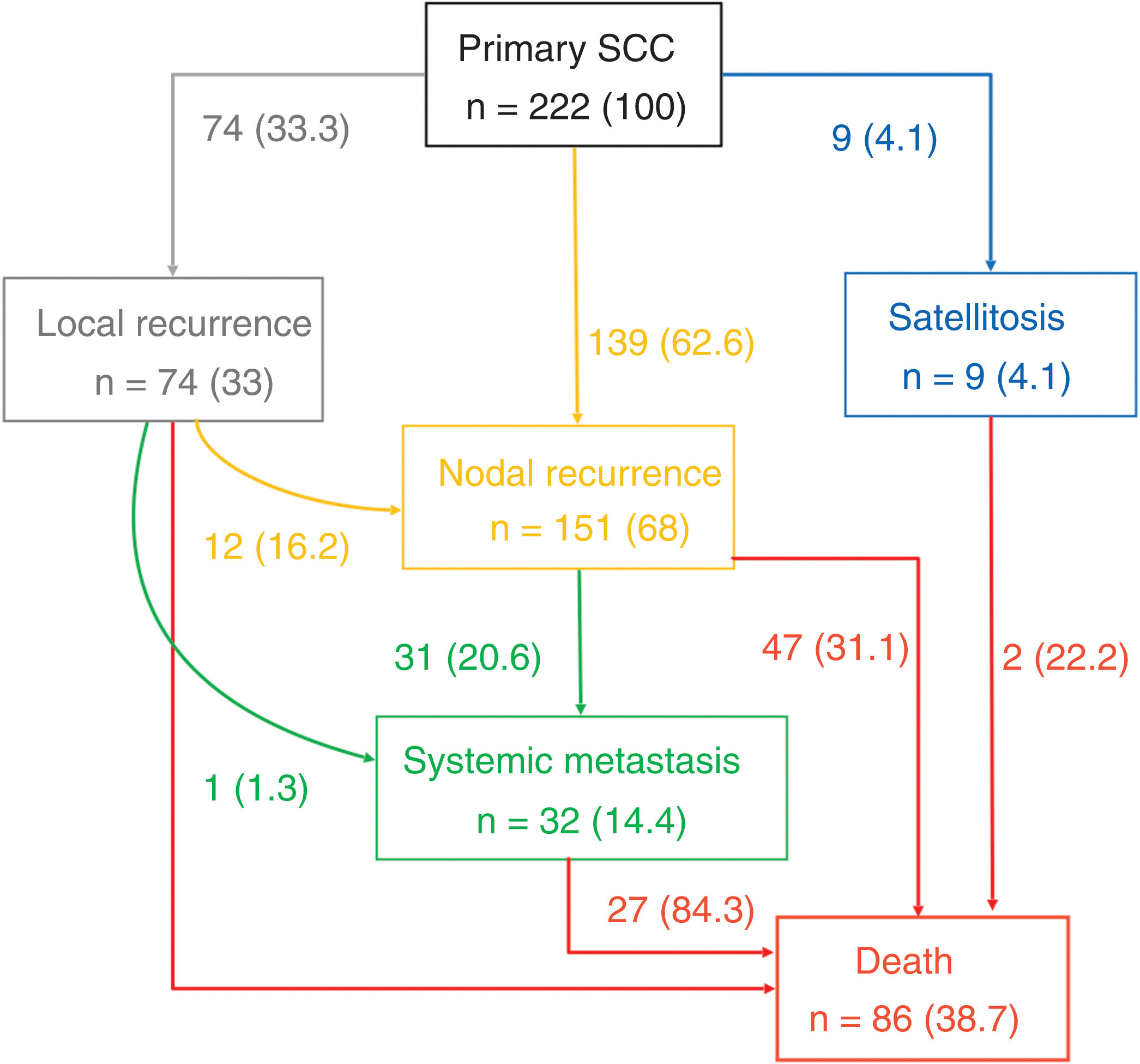
Figure 1 illustrates the different metastatic pathways in the progression of primary cSCC. The most frequent progression pathway was lymphatic (n=139 [62.6%]), followed by local recurrences (n=74 [33.3%]) and satellitosis (n=9 [4.4%]). In 12 cases of local recurrence (16.2% of LR), subsequent lymphatic progression was reported.
Type of relapse and different metastatic pathways of the 222 patients with high-risk cSCC with relapses at their follow-up. (*) indicates percentage (%) of patients, in nodes it is the % over the total number of patients with recurrence, and in the arrows it indicates the % over the upper node.
Overall, 31 cases (20.5% of L) out of all cases with regional lymphatic recurrence progressed into systemic metastases. Only 1 case of local recurrence was described, which directly developed systemic metastases. Systemic metastases were not found as the first progression pathway, nor were lymphatic metastases following satellitosis.
The deaths of patients with cSCC were most due to the regional lymphatic progression of their disease (n=47 [54.6% of deaths]), followed by systemic metastases (n=27 [31.4% of deaths]). Local progressions were the cause of death in 11.6% (n=10) of all reported deaths. Finally, 2 deaths due to satellitosis were reported (2.3% of all reported deaths).
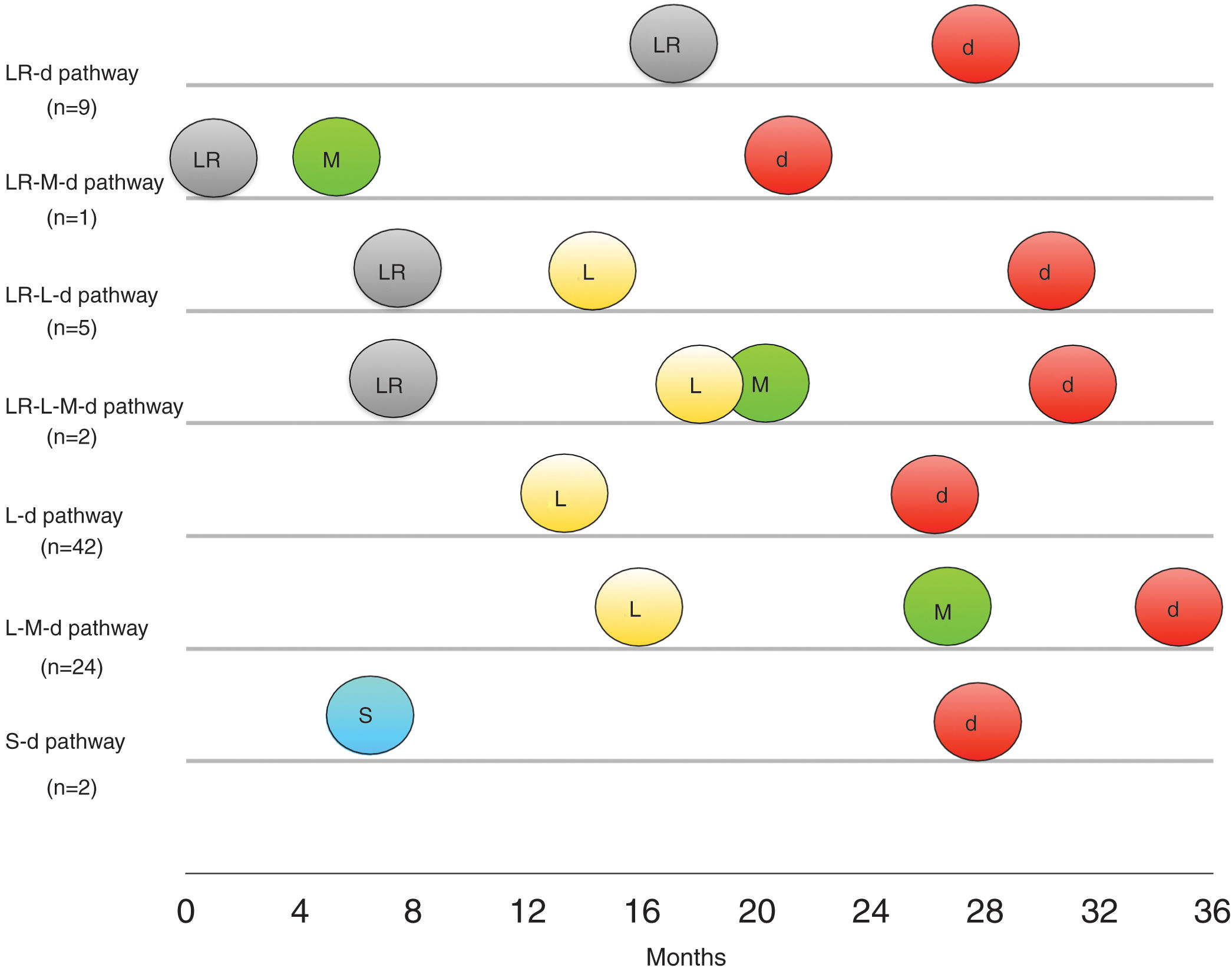
The different metastatic and progression pathways of cSCC cases resulting in death are depicted in Figure 2. The most frequent metastatic pathway was the L-d route (n=42 [49.4%]), followed by the L-M-d route (n=24 [28.2%]). The next most frequent route was the LR-d route (n=9 [10.6%]). The remaining metastatic and progression pathways occurred in 5 or fewer cases. Of note that, regardless of the metastatic pathway involved, all regional lymphatic recurrences appeared, on average, between the 13- and 18-month follow-up, while disease-related deaths occurred, on average, between the 26- and 35-month follow-up from diagnosis for the most frequent metastatic pathways. Interestingly, there was a trend toward longer time to death in cases with L metastasis followed by development of systemic metastases (L-M-d route) compared with cases that only had local progression (LR-d route), via satellitosis (S-d route), or regionally (L-d route).
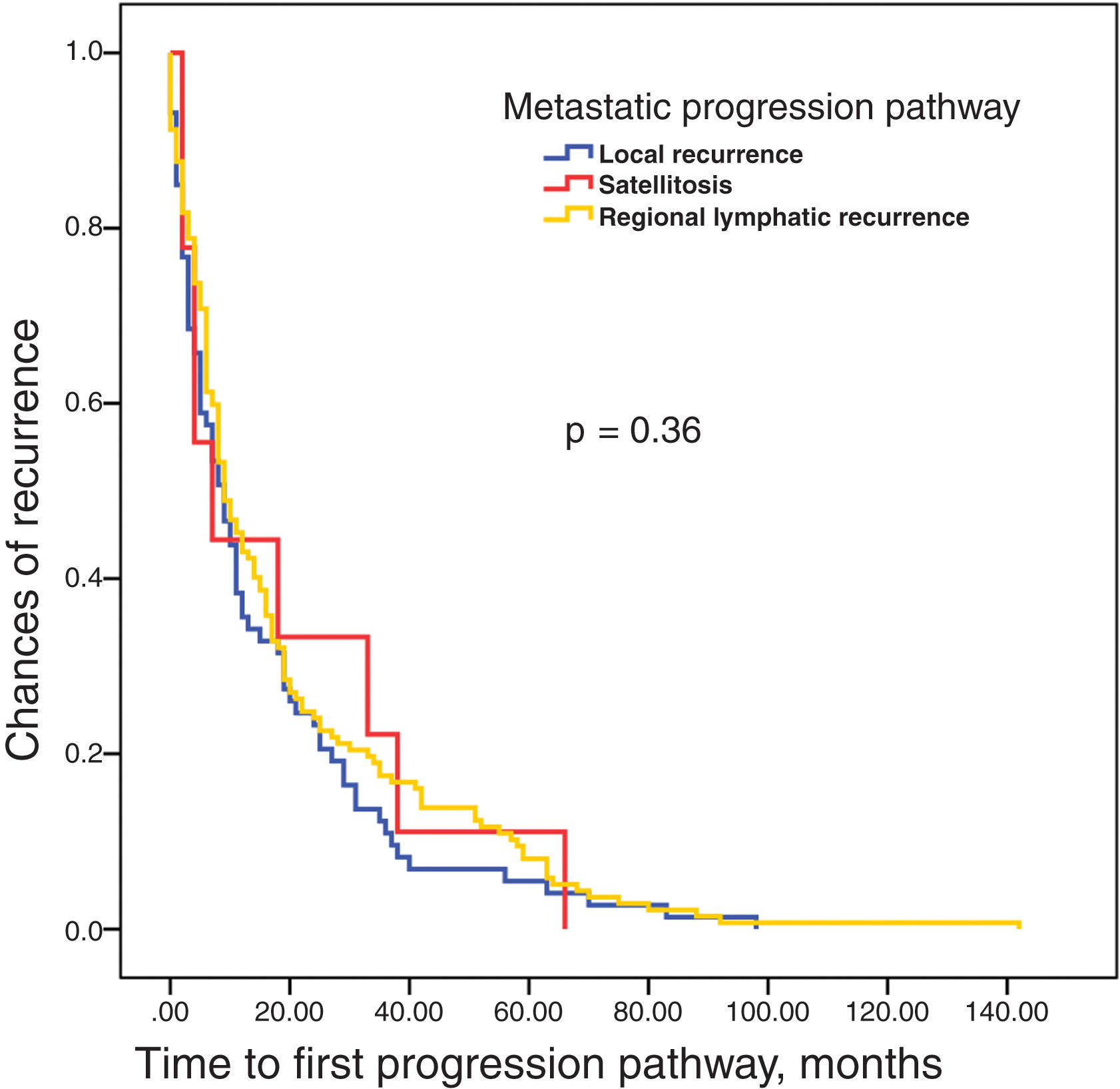
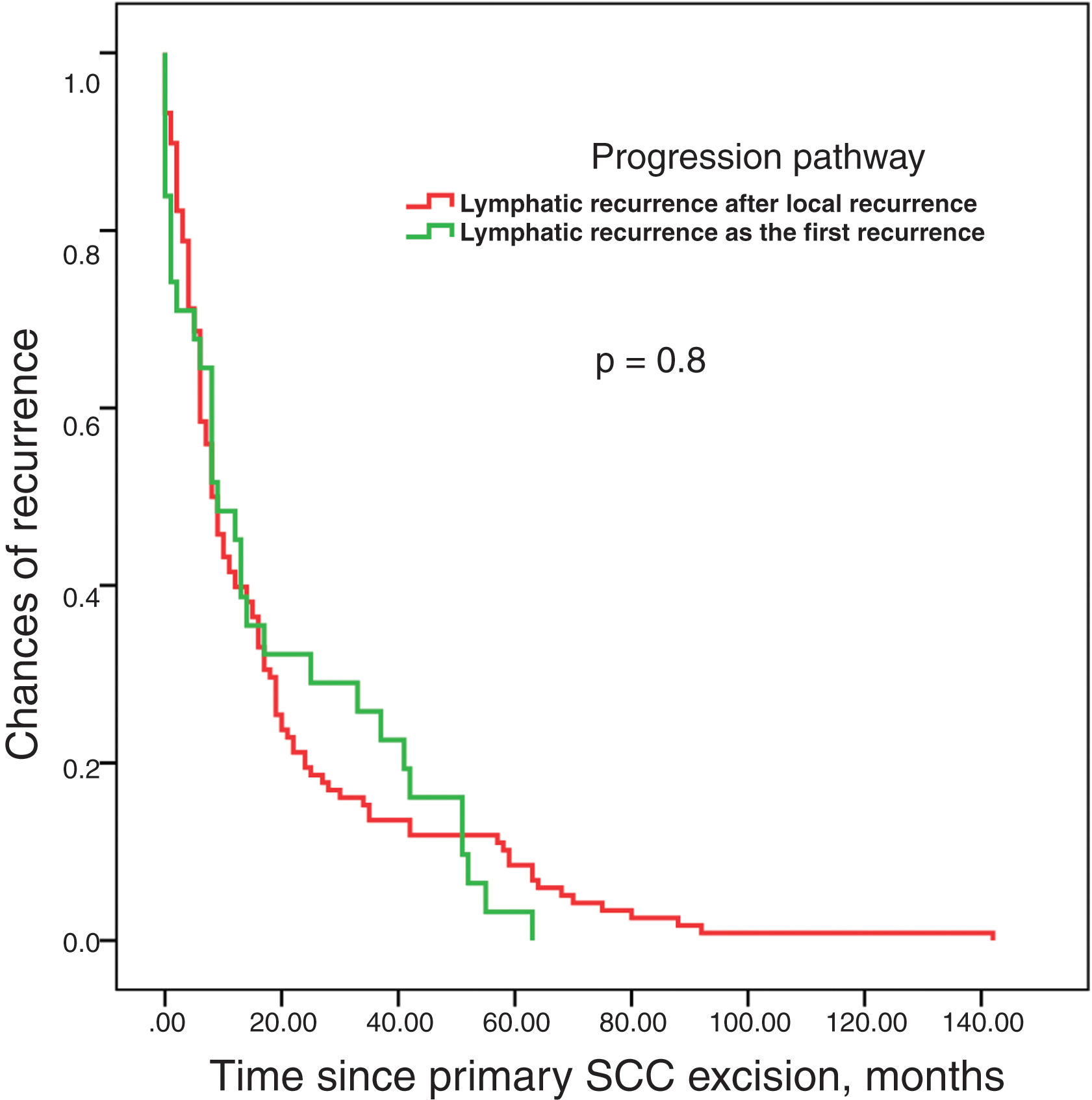
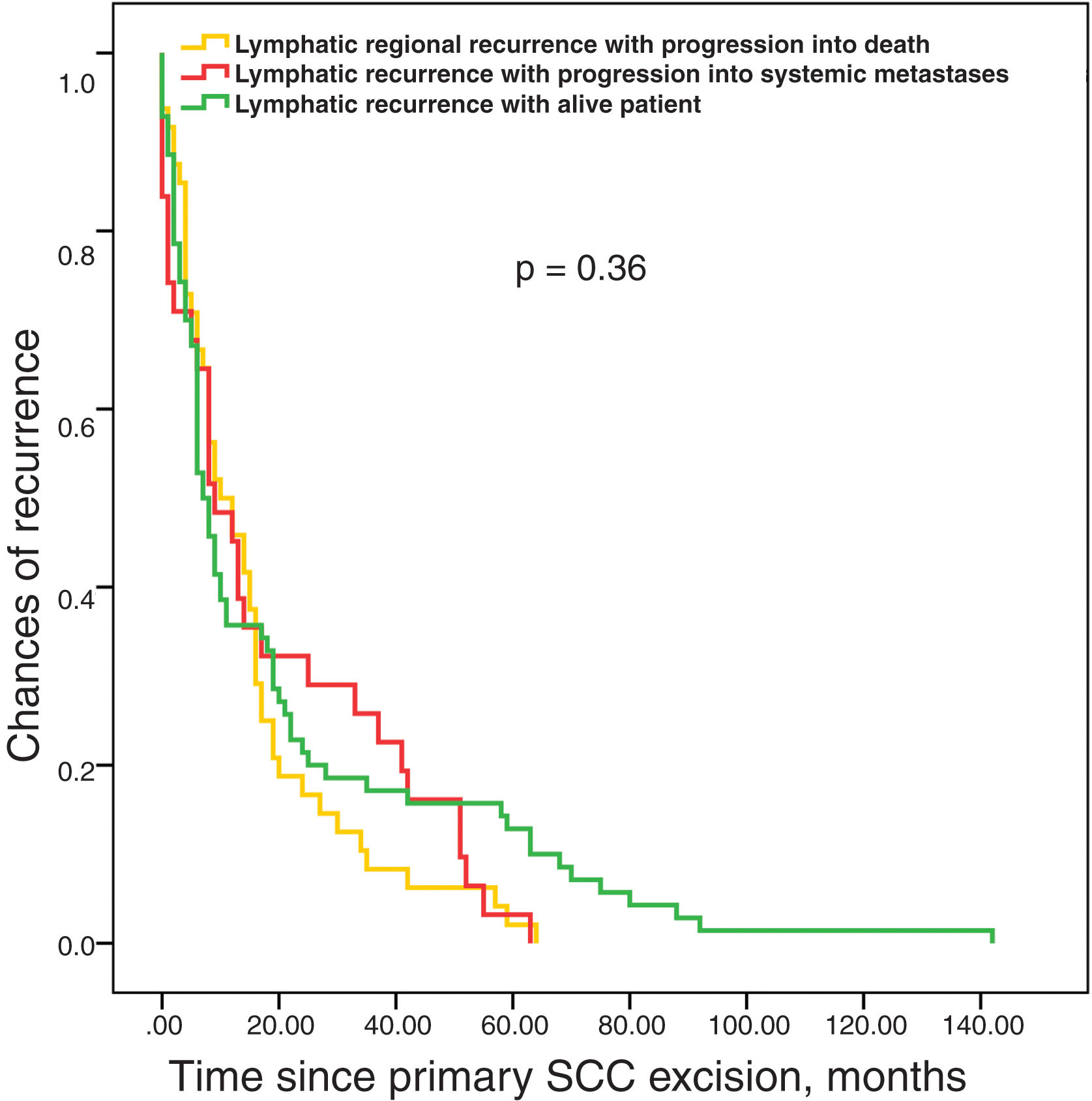
Survival analysis until recurrence based on the type of pathway (LR, L, and S) showed no differences among them (figure 3). No differences in survival time were reported either until the appearance of lymphatic metastases based on whether they were the first recurrence or followed local recurrence (figure 4). Finally, the time elapsed until the appearance of lymphatic metastases was not associated with prognosis, which was determined as the absence of further progressions vs progression to systemic metastases or regional progression until the patient's death (figure 5).
The present study shows that the most frequent progression for high-risk cSCCs is the lymphatic pathway, and that this pathway is virtually mandatory before systemic metastases develop.
The metastatic process is a complex process in which genetically unstable cells adapt to a tissue microenvironment, which is different from their own tissue of origin.12 This process is not random; tumor cells generally have a certain affinity for specific tissues depending on the tumor lineage in a dissemination model originally termed “seed and soil” by Paget back in the 19th century.13 This model explains why certain tumors, such as breast cancer, frequently metastasize to the lung, bone, liver, or brain, while others, such as prostate cancer, are more prone to bone metastasis. Similarly, uveal melanoma characteristically metastasizes more to the liver, while sarcomas metastasize to the lung.12
In the case of cSCCs, the most important implication of these findings would be the locoregional management of the disease. More than half of the patients in the study die from progression of regional lymphatic metastases, which raises the question of whether elective lymph node dissection could benefit these patients, as in the case of oral cavity squamous cell carcinoma.14 However, available observational studies present conflicting results. Amit et al.15 did not demonstrate the benefit of elective lymph node dissection in cSCC patients compared with observation. Conversely, in a different observational study, Xiao et al.16 did observe improved survival in patients undergoing elective lymph node dissection and superficial parotidectomy.
The ordered and gradual progression of cSCC from the primary tumor to the regional lymph node and from there to metastatic dissemination is the ideal theoretical basis supporting the therapeutic use of sentinel lymph node biopsy (SLNB). This is was decades ago was called the “incubator hypothesis”17 by SLNB advocates in melanoma, compared to the “marker hypothesis,” which hypothesized that the presence of metastases in regional lymph nodes is not a preceding step, but a marker that the tumor has already spread to other organs.18,19 The observation that the median survival time is longer for metastatic pathways involving the appearance of systemic metastases (figure 2) vs the same pathways without systemic dissemination to some extent supports this “incubator hypothesis,” an unseen phenomenon in similar studies for melanoma.8,9
Paradoxically, while a clinical trial has evaluated the role of SLNB in melanoma,3 showing no survival benefit, no clinical trial has ever been conducted evaluating the role of SLNB in cSCC. However, observational studies have been conducted comparing cSCC patients undergoing SLNB with patients under observation alone without any survival differences being reported. In this line, our group has found differences in a recent observational trial when stratified by patient immune status, with a clear advantage in progression-free survival seen only in immunocompetent patients undergoing SLNB (unpublished data).
The problem with the indication of this technique, and the overall prediction of lymphatic and systemic metastases, is far from resolved, as only 4% to 5% of patients will develop lymphatic metastases, and of those, only a quarter will develop systemic metastases. In a recent systematic review, SLNB positivity in cSCC was 8%, without clear predictors of involvement identified just yet.4 Current evidence on genetic changes in cell cycle regulation, tumor suppression, tissue invasion, microenvironment, and immune system interaction is still far from offering specific targets that can more accurately predict which patients will progress.20
In our series, 11.6% of specific deaths were due to local progression. These data demonstrate the importance of local control of cSCC. In a recent systematic review, deep invasion beyond fat, or to a lesser extent, desmoplasia, are the histological factors that most predict this recurrence,21 with Mohs surgery being the preferred option for local control of cSCC.21 In a recent review, the success of SLNB in cSCC was not affected whether performed synchronously or asynchronously with surgery, including Mohs surgery.22 This is important in the current context, in which Mohs surgery is becoming increasingly common, and the SLNB would be required after the procedure.23
In conclusion, the most frequent metastatic dissemination pathway for cSCC is the lymphatic pathway. This pathway is virtually necessary for systemic dissemination. Regional progression of lymphatic metastases is also the main cause of death. Measures aimed at regional control of this tumor, such as SLNB, elective lymph node dissection, or adjuvant radiotherapy, could play a role in improving survival in this disease.
FundingThis study was funded by Fundación Piel Sana of the Spanish Academy of Dermatology and Venereology. JC's research has been partially funded by Instituto de Salud Carlos III, Madrid, Spain (and co-funded by the European Union) through project PI21/1207, by the Regional Health Management of Castilla y León, Spain (GRS2549/A/22).
Conflicts of interestNone declared.