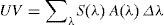Vitamin D enhances musculoskeletal health and reduces mortality related to bone disease in some populations, particularly the elderly and other high-risk groups. Evidence suggests that vitamin D has an impact in cancer, cardiovascular disease, autoimmune processes, and infections. Epidemiologic studies have also detected vitamin D deficits or insufficiency in nearly all the world's populations. Such evidence has led to debate related, to a certain degree, to photoprotective measures that aim at protecting against skin cancer. The latest recommendations of the American Institute of Medicine consider that serum levels of 20 ng/mL (50 nmol/L) appear to be adequate in the general population and achievable even with minimal sun exposure. If these figures are reliable, the apparent pandemic of vitamin D deficiency reported in recent years may be exaggerated. This article reviews the evidence and issues under discussion, looking especially at the role ultraviolet radiation plays in synthesizing vitamin D in the skin. The conclusion is that sun exposure should not be used as the only source of vitamin D given that it is also clearly carcinogenic for the skin. A healthful approach combines moderate sun exposure, adequate food sources of the vitamin, and supplements whenever required.
La vitamina D aumenta la salud musculoesquelética y reduce la mortalidad asociada a problemas óseos en algunos grupos de población, especialmente en los ancianos y otros grupos de riesgo. Existen evidencias de que la vitamina D influye en el desarrollo del cáncer, la enfermedad cardiovascular, los procesos autoinmunes y las infecciones. Por otro lado, distintos estudios epidemiológicos avalan un estado de deficiencia o insuficiencia de vitamina D en la población de casi todo el mundo. Ante todas estas evidencias surgen diferentes controversias, en parte relacionadas con las medidas de fotoprotección impulsadas para prevenir el cáncer cutáneo. Las últimas recomendaciones del Instituto de Medicina Americano (IOM) consideran que niveles séricos de 20ng/ml (50nmol/l) parecen suficientes y alcanzables para la población general, incluso en condiciones de mínima exposición solar. Si atendemos a estas cifras, quizás la prevalencia de esa hipovitaminosis casi pandémica comunicada en los últimos años esté sobreestimada.
El presente artículo recoge todas estas evidencias y controversias, además de profundizar en el papel de la radiación ultravioleta en la síntesis cutánea de la vitamina D. La conclusión es que no se debe tomar el sol como fuente primordial de vitamina D, puesto que se tiene certeza de que la radiación ultravioleta es un carcinógeno cutáneo. Lo saludable es combinar una exposición solar limitada junto a una adecuada alimentación y la administración de suplementos cuando sean necesarios.
Vitamin D is known as “the sunshine vitamin”, which is, strictly speaking, a misnomer because the term vitamin denotes an organic compound required by the body that must be obtained in small quantities from nutrients. Since living organisms can synthesize vitamin D upon exposure to sunlight, it is in fact a true hormone.1
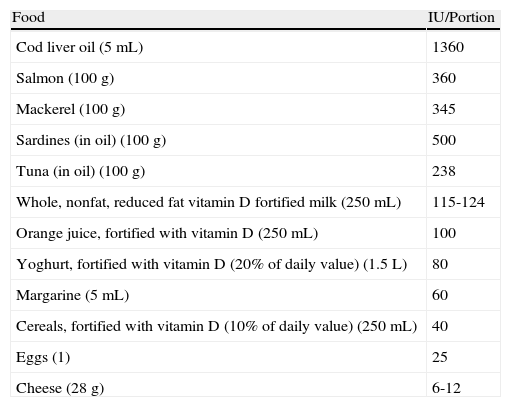
Although we obtain most of the vitamin D we require through exposure to sunlight, a balanced and healthy diet is also essential. Unfortunately, very few foods contain vitamin D, and many of those that do are not commonly consumed (Table 1). This is one of the principal reasons why vitamin D deficiency has reached epidemic proportions in our civilization. Another factor is our indoor lifestyle characterized by unhealthy, sporadic, and intense exposure to sunlight. In addition, the migration of human populations has led a situation in which people with darker skin are living at high latitudes, thereby increasing their risk of vitamin D deficiency, and fair-skinned people are living closer to the equator, where they have an increased risk of skin cancer. One result of this situation is a population characterized by a vitamin D deficiency and, paradoxically, endemic skin cancer. A number of ecological studies have demonstrated geographical variations in the prevalence of several diseases, including certain types of cancer,2 inflammatory bowel disease, multiple sclerosis (MS),3 rheumatoid arthritis,4 type 1 diabetes, and, of course, osteoporosis, with higher prevalence in more northerly latitudes and lower prevalence in southern regions.5 These observations have given rise to the hypothesis that a lack of vitamin D might be implicated in the pathogenesis and prognosis of these diseases.6
Foods Rich in Vitamin D.
| Food | IU/Portion |
| Cod liver oil (5 mL) | 1360 |
| Salmon (100 g) | 360 |
| Mackerel (100 g) | 345 |
| Sardines (in oil) (100 g) | 500 |
| Tuna (in oil) (100 g) | 238 |
| Whole, nonfat, reduced fat vitamin D fortified milk (250 mL) | 115-124 |
| Orange juice, fortified with vitamin D (250 mL) | 100 |
| Yoghurt, fortified with vitamin D (20% of daily value) (1.5 L) | 80 |
| Margarine (5 mL) | 60 |
| Cereals, fortified with vitamin D (10% of daily value) (250 mL) | 40 |
| Eggs (1) | 25 |
| Cheese (28 g) | 6-12 |
Adapted from: http://dietary-supplements.info.nih.gov/factsheets/vitamind.asp#h3.
Office of Dietary Supplements, National Institutes of Health, USA. United States Department of Agriculture, Agricultural Research Service.
USDA Nutrient Database for Standard Reference, Release 21, 2009, Table 3.
However, this hypothesis raises many questions for the dermatologist. Is the exposure to sunlight being recommended to the population really healthy? And what kind of sun protection measures should we recommend to our patients who have skin cancer? Do children and adolescents have adequate vitamin D levels to ensure proper development? Should vitamin D supplements be recommended to certain groups in Spain?
The aim of this article is to review the evidence concerning the effects of vitamin D on health and to make a critical assessment of the controversies involved. Our objective is to propose appropriate strategies for the people living in Spain, a population that has specific characteristics that differ from those of the English-speaking countries where most studies on this topic have been carried out.
Evidence Concerning Vitamin DA Little HistoryVitamin D is a ancient evolutionary advance, and the phytoplankton and zooplankton that have existed in the oceans for 500 million years produce vitamin D when exposed to sunlight.7
The precursor provitamin D (ergosterol or 7-dehydrocholesterol) is incorporated into the plasma membrane lipid bilayer. During the production of previtamin D as a result of exposure to solar UV-B radiation, its ring opens, giving rise to a less rigid open structure and making the membrane more permeable to various ions, including calcium. This may be the reason why vitamin D has always been so important in the regulation of calcium metabolism and ultimately in the evolution of the life forms that have developed endoskeletons and taken to life on land.5
The first evidence of the importance of sunlight to human health emerged during the industrial revolution in northern Europe. People moved into cities, where they lived in polluted and overcrowded conditions and suffered food shortages. Glissen, DeBoot, and Whistler were the first people to report growth retardation and skeletal deformities, which they called rickets, in children living in these northern European cities.5 In 1822, Sniadecki was the first scientist to attribute the development of this disease in children to a lack of adequate exposure to sunlight.8 In 1921, McCollum identified a substance present in certain fats that could prevent rickets. As it was the fourth vitamin to be discovered it was called vitamin D. In 1922, Hess9 reported that daily exposure of children to sunlight on the roof of his hospital in New York for a period of several months was an effective treatment for rickets.
From 1930, various food products in the USA and Europe were fortified with vitamin D. However, after World War II, a lack of proper monitoring of this process led to excessive supplementation and an outbreak of vitamin D intoxication among children and young people,10 an episode that led to a ban on the vitamin D fortification of dairy products in most European countries.11 Today, only a few dairy products in Spain are fortified with vitamin D.
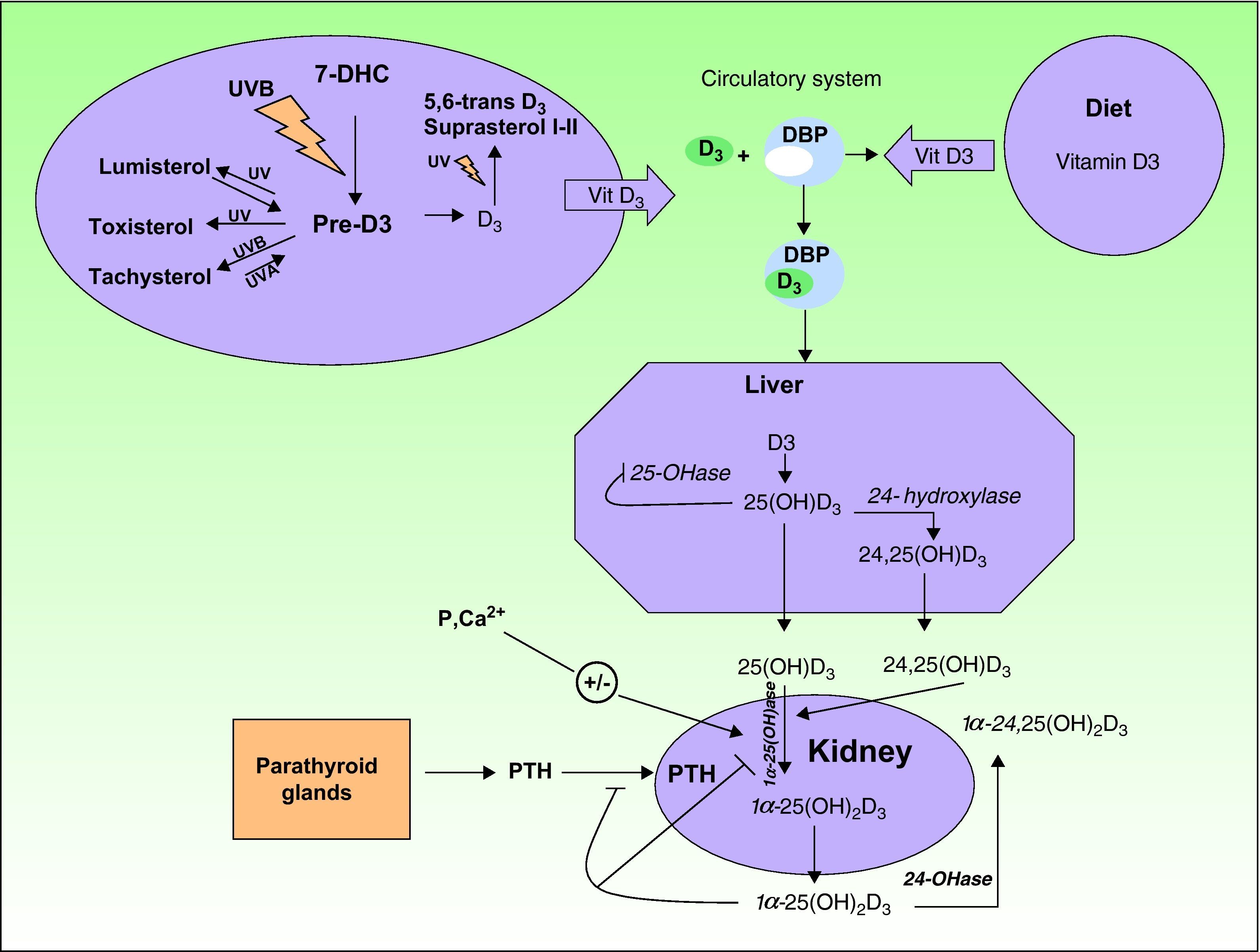
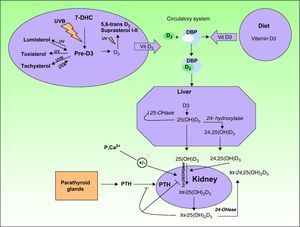
Biosynthesis of Vitamin DThe term vitamin D refers to 2 molecules (vitamin D2 and vitamin D3) that differ in both structure and origin.
- –
Vitamin D2 or ergocalciferol is formed by the action of UV radiation on the steroid ergosterol in plants.
- –
Vitamin D3 or cholecalciferol is synthesized in the skin when UV radiation is absorbed by 7-dehydrocholesterol (Fig. 1).
Upon exposure to sunlight, 7-dehydrocholesterol (also called provitamin D3) absorbs UV radiation and is converted to a compound called previtamin D3. This is the first step in the formation of vitamin D3 or cholecalciferol in a thermochemical reaction triggered by the excited state of 7-dehydrocholesterol. In a series of photoreversible reactions triggered by the absorption of UV-B and UV-A photons, previtamin D3 can also be transformed into other derivatives, such as lumisterol and tachysterol. Likewise, the absorption of photons by vitamin D3 can also result in the formation of 5,6-transvitamin D3 and suprasterol 1 and 2 (Fig. 1). Vitamin D3 is secreted into the extracellular space, where it is transported in the bloodstream by the vitamin D binding protein to the liver. Within the liver, it is hydroxylated through a series of enzyme reactions to 25-hydroxyvitamin D3 or 25(OH)D3, also called calcitriol. A second hydroxylation, catalyzed by the enzyme 24-hydroxylase, can give rise to the 24,25(OH)D3 form. Calcitriol, the main circulating form of vitamin D3, is later hydroxylated in the kidney by the enzyme 1-hydroxylase to its more active form, 1,25-hydroxyvitamin D3 (1,25(OH)D3). The production of all these active forms is regulated by the metabolism of calcium, phosphorus, parathyroid hormone, and magnesium.
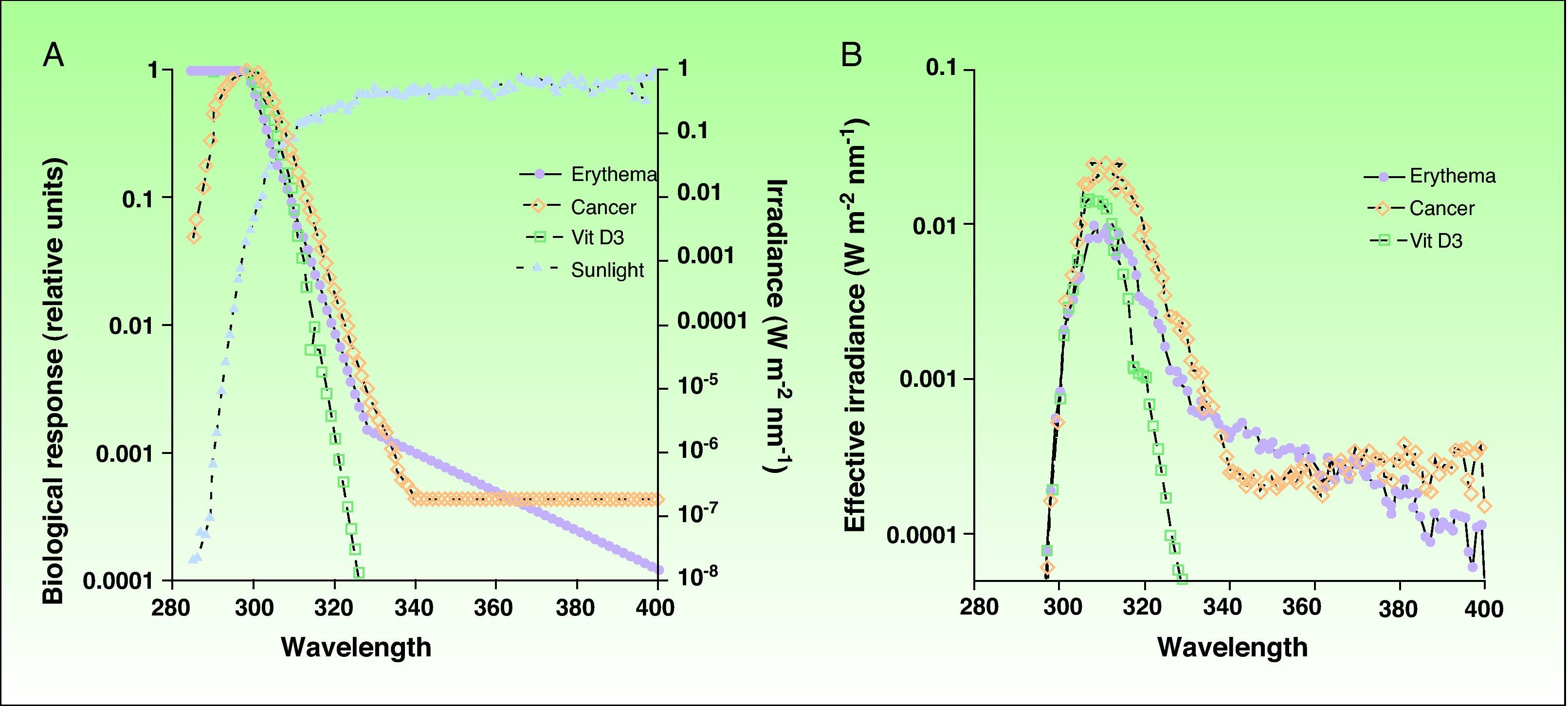
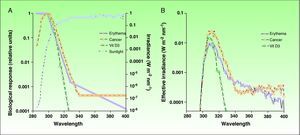
Ultraviolet Radiation: Action Spectrum and Biological ResponsesThe most effective UV radiation spectrum for the formation of previtamin D3 is between 295 and 330nm (Fig. 2).12,13 However, this spectral band is also associated with negative effects, such as sun-induced erythema and squamous cell carcinoma, 2 entities with a common pathogenesis, namely, damage to cell DNA. The action spectra for other types of skin cancer, such as basal cell carcinoma and melanoma, have not yet been clearly defined, although the role of UV-A radiation is considered to be clinically significant.14,15 Since exposure to harmful solar radiation is needed to produce the required levels of provitamin D3, the risk-benefit ratio of such exposure must be more precisely defined, particularly because there are variations (primarily in the UV-B band) in the doses of radiation that produce each one of these biological effects. The potential negative repercussions of such exposure is one of the key issues in the controversy surrounding recommendations of total photoprotection and the risks associated with previtamin D3 deficiency. However, if we can determine the levels of radiation that will produce each of these biological effects, we will be able to define with greater precision a dose that will provide an acceptable balance between photoprotection and beneficial exposure to sunlight.
A) Action spectra associated with erythema, nonmelanoma skin cancer,and the production of vitamin D3 with respect to the spectrum of incident solar radiation during the midday hours. B) Effective spectra of solar irradiation for the 3 biological effects. Vit D3 indicates vitamin D3.
Source: McKinlay AF et al,12 De Gruijl FR et al,13 and Erythema Reference Action Spectrum and Standard Erythema Dose.21
Firstly, taking into account the spectrum of incident solar radiation and the action spectra, we can calculate the effective dose of solar radiation required to achieve different biological effects in a particular place and at a particularly time of day, since incident solar radiation varies according to time and place. A biologically effective level of radiation is the result of the sum, in the entire UV spectrum,16 of all the products of the solar radiation spectrum and the relative action spectrum for each wavelength in this band, as expressed by the following formula:
Where Δλ is the wavelength interval (290-400nm), Σ(λ) is the solar irradiance at a given wavelength, and A (λ) is the action spectrum.Owing to the similarity between the action spectra for erythema and vitamin D production, most authors have to date estimated the solar irradiance effective for vitamin D production and erythema to be more or less equivalent. This makes it possible to calculate the doses of radiation required to produce the minimum threshold dose of vitamin D by using the minimal erythema dose. Thus, the aim has been to simplify the formula for calculating the vitamin D effective dose of solar radiation in relation to the erythemal dose because standard patterns of incident radiation, such as the UV index and different skin phototypes, have already been established based on erythema.
However, there are limitations to formulas based on biologically effective radiation levels, and the solar irradiance required to produce vitamin D must be established independently of the erythemal dose because the action spectrum is similar but not identical. In the spectrum for vitamin D synthesis, UV-B radiation is more heavily weighted, while the spectrum for erythemal action also includes UV-A radiation. This is a very important distinction because solar radiation varies qualitatively as well as quantitatively according to time and place; thus, there is no simple unvarying relationship between the different biological action spectra. For example, the percentage of UV-B radiation in total UV radiation varies at sunrise and sunset because of changes in the angle of the sun's rays in the same way as this proportion varies according to the season and is different in winter and summer. Furthermore, changes in the ozone column also give rise to variations in the ratio of UV-B with respect to total UV radiation. Comparing the irradiance required to produce vitamin D with the UV index, a linear relationship has been shown when the UV index is greater than 5.5. However, the relationship is not linear for the lower UV index values found in winter. These discrepancies have recently been analyzed, and researchers are already starting to use models that correlate vitamin D effective radiation and the UV index, taking into account factors such as ozone levels and solar zenith angle.17 There are, however, inconsistencies in our understanding of the photochemical production of vitamin D in the skin. Some studies show that for the intensities of UV radiation found at mid-latitudes in the winter, less than 1 hour of full body exposure to the sun would produce sufficient vitamin D.18 However, this finding contradicts our previous belief that vitamin D is not produced in the skin under such conditions.
Therefore, more precise definition of these action spectra, both the biologically effective levels of radiation that will produce skin disease and the levels required to produce vitamin D, would make it possible to optimize the exposure of human skin to UV radiation while guaranteeing adequate levels of vitamin D at minimal risk.
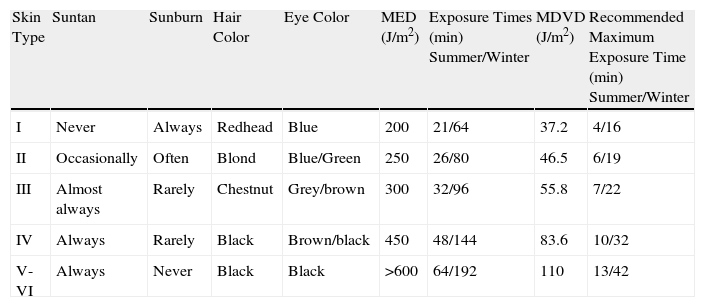
Factors That Influence the Synthesis of Vitamin D in the SkinDaily Cycle of Exposure to SunlightThe biologically effective dose of radiation for the production of vitamin D has a daily cycle, with values peaking at noon and tapering gradually after midday. This variation is directly related to the solar zenith angle19 and the dramatic reduction at dawn and dusk in the percentage of shorter UV-B wavelengths reaching the Earth because these are precisely the wavelengths that have a greater weight in the production of vitamin D. Table 2 shows the exposure to sunlight needed in Spain to obtain optimal vitamin D levels. It indicates the exposure times for each skin phototype required to generate erythema and produce vitamin D during the midday hours in the coastal areas of southern Spain.20 Minimal erythema doses21 have been compared to the standard vitamin D dose, defined as the UV equivalent of an oral dose of about 1000 IU of vitamin D, an intake that guarantees sufficient levels of vitamin D in the blood for this hormone to carry out its function.6 The dose of UV radiation required to produce this standard vitamin D dose is calculated on the basis of the need for approximately 25% of the minimal erythema dose of UV radiation on 25% of the body surface (hands, arms, and face) and taking into consideration the solar radiation weighted by the time at which the measurement is made. For example, the estimated exposure time needed to obtain a minimal erythema dose during the midday hours in summer (mean values from June to August) is approximately 20minutes, while exposure of the hands, arms and face for only 5minutes will produce a standard 1000 IU dose of vitamin D.
Maximum Exposure Times to Biologically Effective Radiation for Minimal Erythema and Vitamin D Dose During the Midday Hours in Coastal Areas of Southern Spain by Skin Phototype.
| Skin Type | Suntan | Sunburn | Hair Color | Eye Color | MED (J/m2) | Exposure Times (min) Summer/Winter | MDVD (J/m2) | Recommended Maximum Exposure Time (min) Summer/Winter |
| I | Never | Always | Redhead | Blue | 200 | 21/64 | 37.2 | 4/16 |
| II | Occasionally | Often | Blond | Blue/Green | 250 | 26/80 | 46.5 | 6/19 |
| III | Almost always | Rarely | Chestnut | Grey/brown | 300 | 32/96 | 55.8 | 7/22 |
| IV | Always | Rarely | Black | Brown/black | 450 | 48/144 | 83.6 | 10/32 |
| V-VI | Always | Never | Black | Black | >600 | 64/192 | 110 | 13/42 |
Abbreviations: MED, minimal erythema dose; MDVD, standard vitamin D dose defined as the amount of effective UV radiation equivalent to an oral intake of 1000 IU of vitamin D, a dose that guarantees sufficient levels of vitamin D in the blood to fulfill its function.
Source: Fioletov et al.17
As mentioned above, solar radiation varies throughout the year not only in quantity but also in quality, with minimal UV-B values in winter at our latitude. Thus, the mean values of effective irradiance at midday required to produce vitamin D between January and March in coastal areas of southern Spain indicate that approximately 4 times the exposure required in summer to obtain healthy levels of vitamin D is needed during the winter months (Table 2).
LatitudeAs we have seen in the case of daily cycles, low angles of solar elevation lead to a reduction in the bands corresponding to the most energetic portion of the UV-B spectrum and therefore affect the photoconversion of vitamin D, which is dependent on these wavelengths. Thus, at latitudes above 51°, a phenomenon called the vitamin D winter has been reported during the cold months.22 This is characterized by minimum values of effective UVB, which leads to vitamin D production in the population below healthy levels. In summer, at high altitudes, the optimal period of UV radiation for the production of vitamin D is short, and exposure to such radiation is associated with an increased risk of sunburn.22 However, this inverse relationship between vitamin D levels and latitude is not a constant finding.18 A recent meta-analysis of 400 studies found no correlation between 25(OH)D levels and latitude except in people with fair skin.23 A study of postmenopausal women in Europe reported similar findings.24 Factors such as diet and individual differences in the adaptation of the skin to sunlight may affect the correlation between vitamin D and the UV-B solar radiation required to induce vitamin D synthesis. The ecologic and epidemiologic studies that attribute differences in mortality or the incidence of certain diseases to vitamin D levels on the basis of latitude must therefore be corroborated by other data.
Skin PhototypeThe irradiation dose required to produce minimum healthy levels of vitamin D in the skin depends on skin phototype, as does susceptibility to erythema (Table 2). The darker the skin, the greater the dose of sunlight that is required.25 This relationship is very significant because at high latitudes where the levels of sunlight are low, vitamin D levels in the population are inversely proportional to skin phototype.26 This is because melanin pigmentation competes for the active photons needed to produce vitamin D. Hence, one of the most important theories about the migrations that have led to the location of different racial groups at different latitudes is that these may have been determined more by vitamin D levels and the diseases associated with a deficiency of this vitamin than by the prevalence of skin cancer in such populations. However, some recent studies indicate that these low vitamin D levels in the population are more closely linked to sun-exposure behavior than to skin phototype.27 One study has even reported that increases in serum 25(OH)D following exposure to UV-B radiation correlated negatively with baseline levels of 25(OH)D and positively with cholesterol levels, but were independent of skin phototype.28 The influence of all these variables makes it difficult to devise a simple and universal recommendation concerning optimal doses of UV-B exposure to adequately increase vitamin D synthesis without increasing the risk of skin cancer.
Environmental Factors: the Ozone LayerThe UV-B radiation that reaches the Earth's surface is directly dependent on levels of stratospheric ozone, and changing atmospheric levels of this gas determine the lowest wavelengths of the incident spectral band.29 Consequently, the so-called ozone hole or seasonal loss of stratospheric ozone influences the levels of photoconvertible vitamin D in the skin. When levels of atmospheric ozone drop, the spectral bands of incident solar radiation fall below 295nm, strongly influencing the photoconversion of vitamin D. However, these low wavelengths are also highly carcinogenic and, in certain latitudes, this danger has resulted in public campaigns about photoprotection which are giving rise to extreme outcomes that could even be considered to constitute sun phobia.30 The Montreal Protocol has achieved a reduction in levels of ozone-depleting substances in the atmosphere, giving rise to a positive and gradual recovery of the ozone layer.18,31 This recovery is taking place now in the 21st century, although there are still uncertainties concerning its eventual interaction with climate change. A recent study suggested that cloud cover may increase at higher latitudes and decrease at lower latitudes in response to climate change. If this prediction proves to be correct, the phenomenon may have important implications for human health since UV radiation may increase at low latitudes, where such radiation is already high, and decrease at high latitudes, where it is already low.18
This development must be communicated to the population as a positive phenomenon, and people should, therefore, be made aware that greater exposure to natural sunlight may be required in the future to increase vitamin D levels.
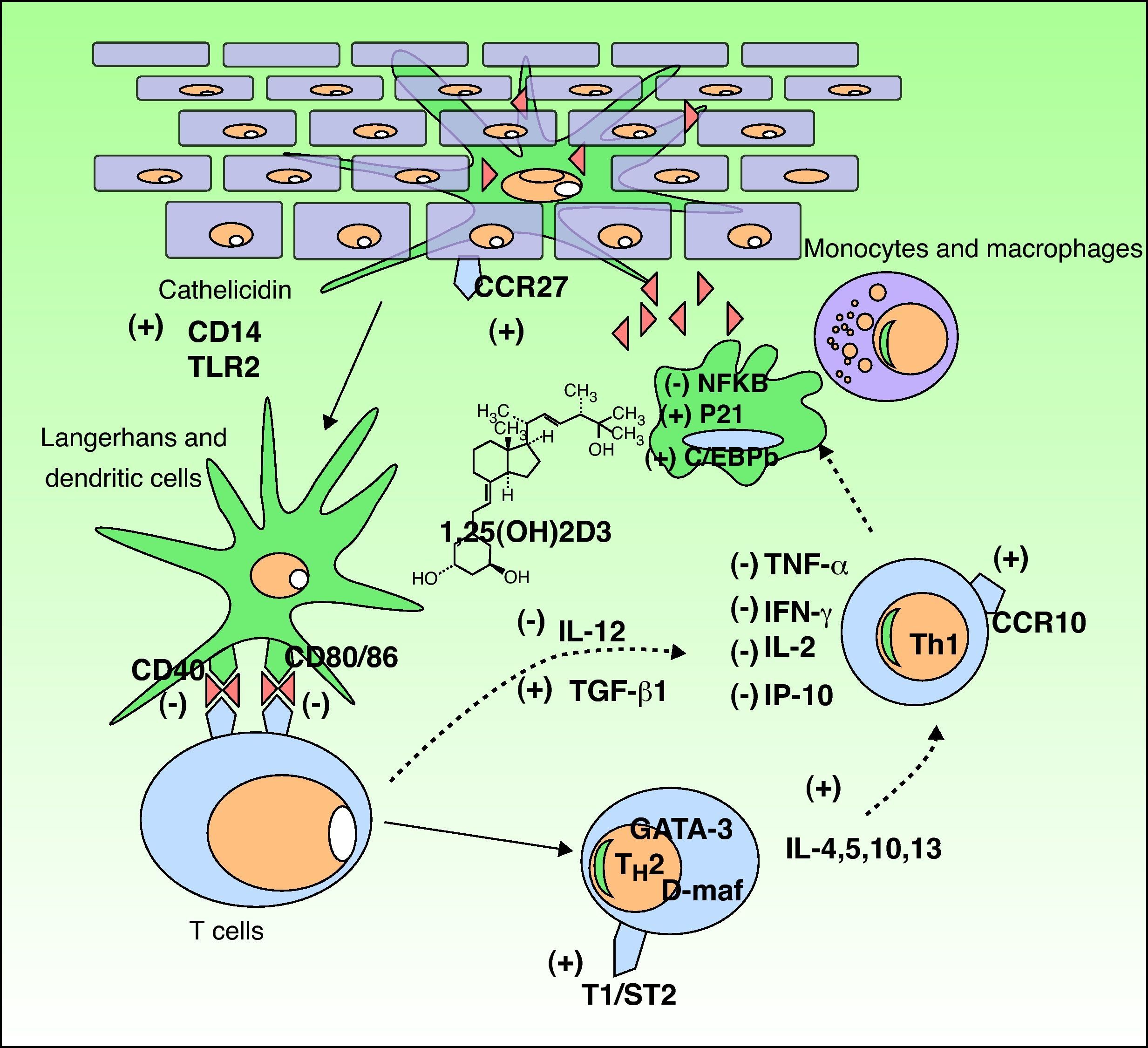
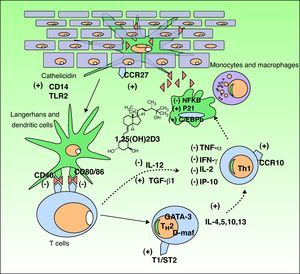
The Functions of Vitamin DVitamin D3 has 2 important functions in the body: the homeostasis of calcium and phosphorus and the modulation of the immune response. We will focus on the latter because it is the function most implicated in many disorders that have recently been linked to vitamin D levels (Fig. 3).
Immunomodulatory effect of vitamin D in the skin. Vitamin D production promotes the innate immune response by activating the cathelicidin type immune receptors CD14 and TLR2 in the epidermal keratinocyte and the CCR27 ligand, which attracts T-cells. Vitamin D also stimulates the differentiation and activation of monocytes and macrophages through induction of p21 and C/EBPb. Conversely, vitamin D reduces the antigen recognition capacity of Langerhans cells, the maturation of dendritic cells, and antigenic presentation (reduction of CD40 and CD80/86 receptors. It inhibits the production of TH1 by downregulating IL-12 synthesis and upregulating that of TGFβ1. Moreover, it partially inhibits the action of TH1 by reducing the production of the cytokines IL-2, IFNγ, TNF-α, and IP-10. In addition, it stimulates the differentiation of TH2 (overexpression of the T1/ST2 receptor of IL-1). Vitamin D also produces overexpression of GATA-3 and D-maf genes, promoting the release of the cytokines IL-4, 5, 10, and 13 in these lymphocytes. CCR indicates carbon catabolite repression; IFN, interferon; IL, interleukin; IP-10, interferon-gamma–induced protein-10; NFKB, nuclear factor kappa B; TGF, transforming growth factor, TLR, toll-like receptor; TNF, tumor necrosis factor; TH, T helper cell.
Following the discovery of vitamin D receptor expression in most of the cells of the adaptive immune system, it has been shown that 1,25D3 inhibits T cell proliferation, cytokine secretion, and cell cycle progression.32 Vitamin D3 can regulate T cells directly and also indirectly through its inhibitory effect on antigen-presenting cells, including dendritic cells. It decreases the secretion of interleukin (IL) 2 and 12, interferon (IFN) γ and tumor necrosis factor (TNF) α, all of which are involved in the T helper (TH) 1 cell pathway (cellular immunity). It also activates certain IL gene promoters in the TH2 pathway.33,34 Furthermore, 1,25D3 is involved in the induction of regulatory T cells and the expression of surface receptors on antigen-presenting cells, such as dendritic cells.32
It achieves this immunologic action by binding to cell receptors,35 thereby activating transcription factors which in turn trigger the following: 1) inhibition of dendritic cells and antigen-presenting cells; 2) a decrease in the production of IL-2, IL-12, TNF-α and IFN-γ; 3) activation of transforming growth factor β, which suppresses the proinflammatory action of TH1 cells; 4) activation of GATA-3 and c-maf genes, which promote the synthesis of TH2 system cytokines (IL-4, IL-5–IL-13); and 5) an increase in IL-10, which in turn inhibits TH1 cells. This immunologic action gives rise to an imbalance that favors the TH2 pathway, and consequently a humoral immune response, anti-inflammatory processes, and immune suppression.36 This enhancement of the TH2 pathwayhas led to the use of vitamin D3 in the treatment of certain autoimmune diseases37; this is discussed below.
Active vitamin D produced in the skin intervenes directly in the skin's innate and acquired immune response and also in its inflammatory response to actinic damage.38 It has been shown that vitamin D can play a crucial role in the intrinsic response to skin cell damage caused by UV radiation.39,40 Studies on human keratinocytes using diverse experimental models have demonstrated the photoprotective effect of calcitriol, an active metabolite of vitamin D, against the production of pyrimidine dimers, as well as a significant reduction in other UV-induced photoproducts.39–41
Vitamin D is also involved in the regulation of antimicrobial peptides, and particularly in the induction of cathelicidin and defensin B4. When an infection or wound activates toll-like receptor 2 (TLR2) in keratinocytes and monocytes, expression of CYP27B1 is induced causing 25(OH)D to convert to its active form 1,25-D3, which results in the induction of cathelicidin and defensin.42 The expression of TLR2 is upregulated by IFN-γ, downregulated by IL-4, and unaffected by IL-17.43
The Link Between Vitamin D Deficiency and DiseaseUntil a few years ago, vitamin D was seen as “the bone vitamin” and was associated with diseases such as rickets, osteomalacia, and osteoporosis because of its key role in bone metabolism. In recent years, however, vitamin D involvement has been demonstrated in practically every medical specialty, including cancer, metabolic syndrome, and infections, as well as numerous autoimmune, metabolic, and neurological disorders, and even pain. This discovery led to the coining of the term “vitamin D insufficiency” to denote the low serum 25(OH)D levels that may be associated with various diseases.44 However, establishing a cutoff point to separate deficiency from insufficiency is problematic because of individual variations in the functional effects of vitamin D and its interaction with calcium intake. Some authors define deficiency as a level of 10 ng/mL or less and insufficiency to be levels between 10 and 20 ng/mL.44
The current evidence supporting the link between vitamin D and the diseases mentioned above is discussed below.
Vitamin D and Bone MetabolismVitamin D plays a critical role in maintaining serum calcium levels, thereby preventing hypocalcemic tetany by stimulating mineralization. Vitamin D maintains serum calcium concentrations through 3 mechanisms. Firstly, it is the only hormone known to be capable of inducing the proteins involved in the intestinal absorption of calcium. Secondly, in the absence of any dietary intake of calcium, vitamin D favors the mobilization of the body's reserves in the bone mass by stimulating osteoclastogenesis. Finally, vitamin D, in conjunction with parathyroid hormone, stimulates reabsorption of part of the calcium filtrate in the renal tubule.45 From a clinical standpoint, vitamin D deficiency is a major risk factor for osteoporosis. Two meta-analyses of randomized controlled trials have shown that taking high doses of vitamin D reduced fall risk by 19% and bone fracture risk by between 15% and 29%.46 However, a recent study reported an increased risk of falls in older women who received a high single annual dose of vitamin D (500000 IU).47 These contradictory results may be related to the differences in the dose regimens used, and the benefit may be more closely associated with the regimen involving more frequent administration than with the total dose administered. However, given that the serum levels of 25(OH)D reached in that study can be obtained with other regimens, further studies are needed to establish the optimal dose and regimen of vitamin D for bone metabolism.
Vitamin D and Autoimmune DiseasesThe involvement of vitamin D in the immune response, discussed above, provides sufficient grounds for the thesis that some of the most prevalent autoimmune diseases should be key targets for the beneficial effects of vitamin D.4
Multiple SclerosisA number of epidemiologic findings support the link between vitamin D deficiency and an increased risk of developing MS. While MS is virtually unknown at the equator, its prevalence increases in proportion to the distance from that latitude. Outbreaks of MS typically occur during the winter and spring, the periods corresponding to the months with the lowest levels of UV radiation and, consequently, with lower serum levels of vitamin D. A population study that followed 187 000 patients for between 10 to 20 years found an inverse relationship between vitamin D levels and the incidence of MS. In that study, the prevalence of MS was found to be 40% lower in women with adequate vitamin D levels. However, this association between low vitamin D levels and MS was only found in white patients and no such association was found among black or Hispanic patients, 2 groups in which lower serum levels of vitamin D are typically found.48 Finally, the results of several studies suggest that administering supplemental vitamin D to patients with MS in remission may protect them from relapse.49 However, these findings must be treated with caution because MS is also associated with a reduction in overall nutritional intake, a circumstance that could give rise to deficiencies in vitamin D and other elements. Similarly, MS also leads to a reduction in outdoor activity and consequently in exposure to UV radiation, an effect that will be more marked in areas characterized by scant sunlight. The strongest evidence that vitamin D may be a natural protective factor against MS comes from data obtained from experimental encephalomyelitis, a murine model of MS, in which administration of 1,25-(OH)2D3 inhibited both the onset and progression of the disease.50 Clinical trials designed to confirm these hypotheses, establish the optimal dose, and determine which patients with MS would benefit from vitamin D supplementation are currently underway.
Vitamin D and Diabetes MellitusSeasonal peaks observed in the incidence of diabetes mellitus have been associated with periodic oscillations in vitamin D levels, among other factors.51 A large 4-year prospective multicenter study in 51 regions worldwide revealed an inverse relationship between UV-B radiation in each area and the incidence of type I diabetes mellitus.52 Vitamin D supplementation, at least during childhood, appears to have some value in preventing this disease, and it has been shown after a long follow-up period of up to 30 years that the administration of 2000 IU of vitamin D daily significantly reduced children's risk of developing insulin-dependent diabetes mellitus.53 However, there is currently insufficient evidence to support the therapeutic usefulness of vitamin D supplementation in managing diabetes mellitus.
Vitamin D and PsoriasisThe relationship between vitamin D and psoriasis has been studied since the 1930s. Krafka and Augusta54 and Thacker55 were the first authors to publish evidence of this relationship. In 1985, Morimoto and Kumahara56 accidently discovered that vitamin D3 supplementation improved psoriasis in an isolated case. Attempts to use oral vitamin D to manage psoriasis were limited by the effects of such supplementation on calcium metabolism. At this point, the race began to find new vitamin D analogs with less hypercalcemic activity, and vitamin D derivatives (calcipotriol, tacalcitol) are now a well established topical treatment for psoriasis. The biological action of these drugs includes regulation of epidermal cell proliferation and differentiation, inhibition of angiogenesis, and modulation of cytokine production.57 Consequently, they also have applications in other disease processes that involve altered cell kinetics, including certain types of cancer. Several authors have attempted to determine the relationship between vitamin D levels and psoriasis. In 1990, Morimoto et al58 failed to find greater vitamin D deficiency in psoriasis patients than controls. However, they did detect lower levels of circulating vitamin D in the patients with more severe psoriasis. In recent years, some researchers have focused on the vitamin D receptor. Okita et al59 studied vitamin D receptor polymorphisms in patients with psoriasis. While they found no relationship between such polymorphisms and the type or severity of psoriasis, they did, nonetheless, find a significant correlation between the AA genotype and liver dysfunction in some patients. This association may be an indication that the vitamin D pathway regulates the expression of the metabolic syndrome that accompanies psoriatic disease. Another very interesting line of research is the link between antimicrobial peptides (defensin, cathelicidin) and psoriasis activity.60 The proinflammatory activity of these peptides is inhibited by vitamin D analogs and by UV radiation, which stimulates vitamin D synthesis in the skin.61 These findings shed new light on the pathophysiology of the disease and suggest new therapeutic approaches.
Other Autoimmune DiseasesExperimental and clinical studies have investigated the possible implication of vitamin D in several autoimmune diseases. The results of experiments with animal models of arthritis suggest that treatment with 1,25(OH)2D3 in the early stages of rheumatoid arthritis may prevent disease progression.62 It has been shown that patients with systemic lupus erythematosus have low levels of 25(OH)D. The factors that contribute to these low levels include the pathophysiological mechanisms of the disease itself, the fact that some patients with the disease develop anti-vitamin D antibodies, and the photoprotective measures imposed by the disease.63 Vitamin D supplementation would therefore appear to be advisable in this situation.63
Vitamin D and InfectionsVitamin D is known to be one of the necessary links in the immediate activation of immunity through the toll-like receptors, making it reasonable to posit its involvement in the development of various infections. Indirect evidence of this involvement is the overexpression of the antimicrobial peptide cathelicidin in human monocytes with the addition of vitamin D. Similarly, it has been shown that the addition of 1,25(OH)2D to macrophages infected with Mycobacterium tuberculosis reduces the number of viable bacilli.
These observations in the laboratory appear to be consistent with various clinical findings, such as the fact that levels of both cathelicidin and vitamin D were lower in patients admitted to an intensive care unit with sepsis than in patients without sepsis in the same setting.64 In the same study, cathelicidin messenger RNA acid levels were appreciably lower in African-American patients, who also had lower serum levels of 25(OH)D than the white patients. Furthermore, 25(OH)D supplementation in patients with vitamin D deficiency made the probability of sepsis equal in both groups.65
With respect to respiratory infections, an association has been found between deficient vitamin D levels in young people of military age and a greater number of sick leave days due to infections of the upper respiratory tract, particularly in patients with a history of asthma or chronic lung disease.65,66 The statistical relationship between polymorphisms of the vitamin D receptors and the incidence of respiratory infections also supports the prognostic value of vitamin D in the management these diseases.67
In terms of intervention, a systematic search of the literature for research into the use of vitamin D derivatives in the prevention and treatment of infection yielded around 10 placebo-controlled studies. The conclusion is that while such derivatives may be useful in the treatment of tuberculosis, influenza, and upper respiratory tract infections, there is insufficient evidence to support definitive recommendations68.
Vitamin D in Cardiovascular DiseaseVitamin D and HypertensionVitamin D is implicated in the control of blood pressure through inhibition of the renin-angiotensin system,69 although other factors are probably involved, including the prevention of primary hyperparathyroidism and the control of calcium metabolism. These hypotheses are supported by experimental findings, such as those reported by Li,70 who found that the administration of 1,25 dihydroxyvitamin D [1,25(OH)2D] suppressed renin gene expression in knockout mice lacking the vitamin D receptor. Moreover, hypertension occurring spontaneously in such mice can be reversed with captopril or 1,25(OH)2D.71
In fact, once again studies involving supplementation have produced modest results. A meta-analysis on this subject evaluated 11 randomized controlled trials of varying methodological quality, most of which had enrolled only a small number of patients. The conclusion found by that analysis was a nonsignificant decrease in systolic blood pressure and a significant, but nonetheless slight, reduction in diastolic blood pressure in the groups who received vitamin D with respect to the control group. However, this effect appears to occur only in hypertensive individuals and is not observed in individuals with normal blood pressure.72 This would suggest that the target population that could potentially benefit from the cardiovascular effects of vitamin D would be patients with high blood pressure and a vitamin D deficiency.73
Vitamin D and Congestive Heart FailureThe relationship between vitamin D deficiency and congestive heart failure could, a priori, be linked to the greater prevalence in vitamin D deficient patients of risk factors for developing heart diseases, such as diabetes mellitus and hypertension. However, in recent years, our understanding of the pathophysiology of congestive heart failure has changed from a purely hemodynamic model towards a more complex mechanism that includes the action of TNF and IL-6, proinflammatory cytokines that may be affected by the immunomodulatory effects of vitamin D derivatives.74 Epidemiological evidence supporting that mechanism includes the high prevalence (up to 75% has been reported) of hypovitaminosis D in patients with congestive heart failure and coronary heart disease.75 Higher mortality during the winter months has also been reported in patients with congestive heart failure, coinciding with lower levels of UV radiation.76 The results of experiments in animal models also appear to support the hypothesis. Vitamin D receptors are expressed in cardiomyocytes, vascular smooth muscle cells, and endothelial cells, where vitamin D may be implicated in inflammation as well as cell proliferation and differentiation.77 In a study of murine models using vitamin D knockout mice, histology revealed marked cardiac hypertrophy and collagen accumulation.78 These changes were attributed to the overstimulation of the renin-angiotensin system and of cardiomyocytes, including an increase in contractility. All these abnormalities and changes were reversed by administration of vitamin D for 13 weeks. These results suggest that the action of vitamin D in congestive heart failure goes beyond its implication in the risk factors, because it also intervenes in the regulation of the inflammatory process that accompanies and conditions the disease itself.
However, therapeutic interventions have not as yet achieved the desired results. Vitamin D supplementation in patients with congestive heart failure has been associated with increased levels of the anti-inflammatory cytokine IL-10 and decreased TNF levels, but no repercussion on or correlation with clinical course has been observed.79
Vitamin D and CancerSeveral studies, in both animal models and humans, have provided evidence that vitamin D may have a beneficial effect on cancer in terms of both reducing incidence and improving the survival of patients with such disease. Some studies have reported a negative correlation between some 17 to 21 types of cancer, including melanoma, and the UV-B index in the USA, 80 Canada,81 and Spain.2
The mechanism of action is very probably linked to the regulatory effects of vitamin D on cell growth, differentiation, and death, as well as on angiogenesis.82 The role of vitamin D in carcinogenesis has been assessed by studying the relationship between disease incidence and patient survival in various malignancies and vitamin D levels, polymorphisms in the vitamin D receptor, and dietary vitamin D supplementation.
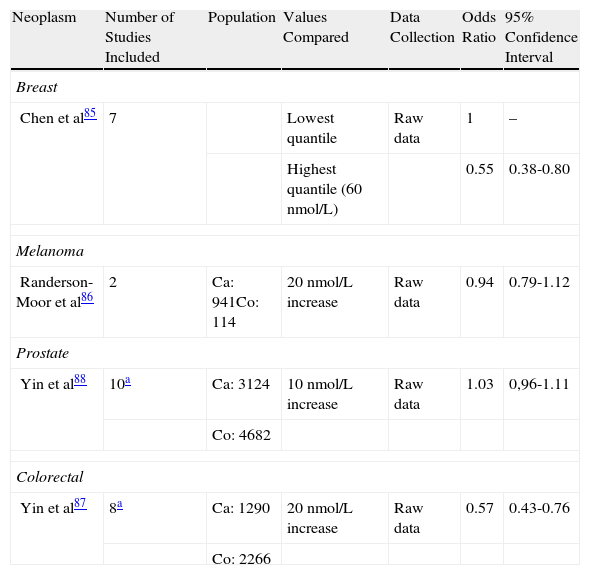
In recent years, many studies have attempted to link blood levels of 25(OH)D with the incidence of various cancers. In these studies, minimum values of 30 to 35 ng/mL (75 to 87.5 nmol/ L) have been used as a reference. These are considered to be the optimal levels for obtaining the maximum beneficial effects of vitamin D.83,84 Recently, 4 meta-analyses have reported conclusive evidence regarding the protective effect of adequate levels of vitamin D against breast and colorectal cancer, but not against prostate cancer and melanoma (Table 3).85–88 This effect persists even after adjusting for factors that might influence vitamin D levels, such as body mass index or age. Some authors have even analyzed the optimal daily intake of vitamin D to achieve protection against cancer. A dose of 1500 IU/d of vitamin D3 (cholecalciferol) has been shown to reduce the mortality rate from cancer in men by 30% in the USA.89
Summary of the Meta-Analyses Assessing the Implication of Serum 25(OH)D2 Levels in the Development of Neoplasms.
| Neoplasm | Number of Studies Included | Population | Values Compared | Data Collection | Odds Ratio | 95% Confidence Interval |
| Breast | ||||||
| Chen et al85 | 7 | Lowest quantile | Raw data | 1 | – | |
| Highest quantile (60 nmol/L) | 0.55 | 0.38-0.80 | ||||
| Melanoma | ||||||
| Randerson-Moor et al86 | 2 | Ca: 941Co: 114 | 20 nmol/L increase | Raw data | 0.94 | 0.79-1.12 |
| Prostate | ||||||
| Yin et al88 | 10a | Ca: 3124 | 10 nmol/L increase | Raw data | 1.03 | 0,96-1.11 |
| Co: 4682 | ||||||
| Colorectal | ||||||
| Yin et al87 | 8a | Ca: 1290 | 20 nmol/L increase | Raw data | 0.57 | 0.43-0.76 |
| Co: 2266 | ||||||
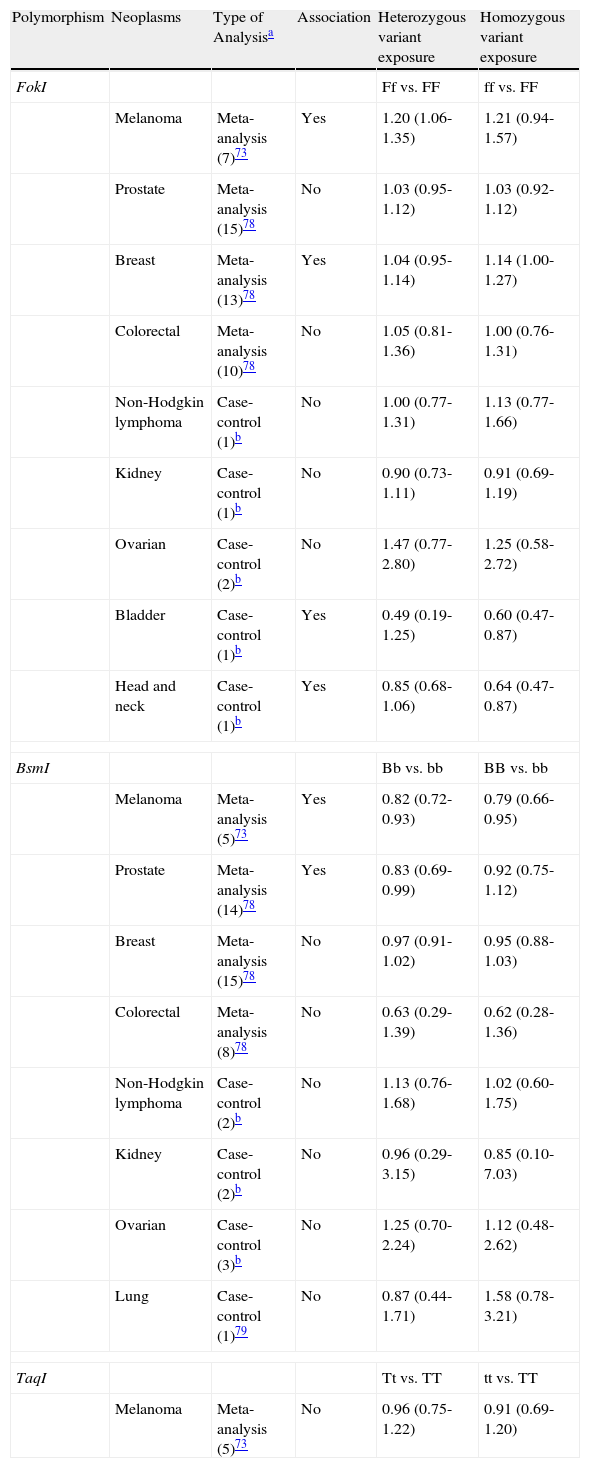
The gene that codes for the vitamin D receptor is located at chromosome region 12q13 and has numerous variants, some of which may alter the function of the receptor. The most studied variant is located at a FokI restriction site (F/f) in exon 2. This variant is associated with an alteration in the gene transcription start codon resulting in a shorter vitamin D protein (F), which is thought to be more active.90 The authors of 2 meta-analyses evaluating the less active f variant reported that it confers a greater risk of developing melanoma or breast cancer, but has no affect on prostate or colorectal cancer (Table 4).86,91 Additionally, several case-control studies have shown that this variant is not associated with increased risk for non-Hodgkin lymphoma, kidney cancer, ovarian cancer, or lung cancer.91 It is interesting to note that the f variant appears to have a protective effect against cancer of the bladder, head, and neck.91
Summary of Studies Evaluating Polymorphisms in the Vitamin D Receptor Gene and Susceptibility to Cancer.
| Polymorphism | Neoplasms | Type of Analysisa | Association | Heterozygous variant exposure | Homozygous variant exposure |
| FokI | Ff vs. FF | ff vs. FF | |||
| Melanoma | Meta-analysis (7)73 | Yes | 1.20 (1.06-1.35) | 1.21 (0.94-1.57) | |
| Prostate | Meta-analysis (15)78 | No | 1.03 (0.95-1.12) | 1.03 (0.92-1.12) | |
| Breast | Meta-analysis (13)78 | Yes | 1.04 (0.95-1.14) | 1.14 (1.00-1.27) | |
| Colorectal | Meta-analysis (10)78 | No | 1.05 (0.81-1.36) | 1.00 (0.76-1.31) | |
| Non-Hodgkin lymphoma | Case-control (1)b | No | 1.00 (0.77-1.31) | 1.13 (0.77-1.66) | |
| Kidney | Case-control (1)b | No | 0.90 (0.73-1.11) | 0.91 (0.69-1.19) | |
| Ovarian | Case-control (2)b | No | 1.47 (0.77-2.80) | 1.25 (0.58-2.72) | |
| Bladder | Case-control (1)b | Yes | 0.49 (0.19-1.25) | 0.60 (0.47-0.87) | |
| Head and neck | Case-control (1)b | Yes | 0.85 (0.68-1.06) | 0.64 (0.47-0.87) | |
| BsmI | Bb vs. bb | BB vs. bb | |||
| Melanoma | Meta-analysis (5)73 | Yes | 0.82 (0.72-0.93) | 0.79 (0.66-0.95) | |
| Prostate | Meta-analysis (14)78 | Yes | 0.83 (0.69-0.99) | 0.92 (0.75-1.12) | |
| Breast | Meta-analysis (15)78 | No | 0.97 (0.91-1.02) | 0.95 (0.88-1.03) | |
| Colorectal | Meta-analysis (8)78 | No | 0.63 (0.29-1.39) | 0.62 (0.28-1.36) | |
| Non-Hodgkin lymphoma | Case-control (2)b | No | 1.13 (0.76-1.68) | 1.02 (0.60-1.75) | |
| Kidney | Case-control (2)b | No | 0.96 (0.29-3.15) | 0.85 (0.10-7.03) | |
| Ovarian | Case-control (3)b | No | 1.25 (0.70-2.24) | 1.12 (0.48-2.62) | |
| Lung | Case-control (1)79 | No | 0.87 (0.44-1.71) | 1.58 (0.78-3.21) | |
| TaqI | Tt vs. TT | tt vs. TT | |||
| Melanoma | Meta-analysis (5)73 | No | 0.96 (0.75-1.22) | 0.91 (0.69-1.20) | |
The second most studied variant is the polymorphism detected using the restriction enzyme BsmI in intron 8 (B/b). Although this polymorphism is considered silent because it does not alter the sequence of the encoded protein, it may affect gene expression through regulation of messenger RNA stability.90 According to 2 recent meta-analyses, the b variant confers an increased risk of developing melanoma and prostate cancer, but not breast or colorectal cancer (Table 4).86,91 Furthermore, in several case-control studies no association has been observed with non-Hodgkin lymphoma, kidney, ovarian or lung cancer.91,92
The relationship found between vitamin D levels and survival rates is of particular interest. A recent retrospective study found an association between high levels of vitamin D3 at the time of diagnosis and thinner tumors.93 It also found an independent protective effect against relapse or death from melanoma. The same authors also observed a clear interaction between vitamin D levels, the BsmI genotype of the vitamin D receptor, and disease-free survival.93 These results suggest the need to establish appropriate clinical guidelines aimed at maintaining optimal levels of vitamin D in patients with melanoma.
The relationship between vitamin D and skin cancer is particularly complex because of their respective interactions with UV radiation. What does appear to be clear is that patients with a history of skin cancer are at higher risk for vitamin D insufficiency, either because of the photoprotective measures they use to avoid a recurrence of their skin cancer or because of other factors that are still poorly understood.94
However, the association between exposure to sunlight and protection from various internal cancers, possibly due to the production of vitamin D, remains controversial. While institutions such as the International Agency for Research on Cancer Working Group on Vitamin D and Cancer have found only limited evidence to support the association between exposure to sunlight and a reduced risk of breast, colon and prostate cancer,95 other researchers disagree and consider that the evidence is more substantial and supports the prevention of cancer through vitamin D production.96
Controversies Surrounding Vitamin DIs There an Optimal Blood Marker for Vitamin D Status and What Are Desirable Levels?A systematic review showed that circulating 25(OH)D is a robust and reliable marker of vitamin D status.97 Researchers have not defined optimal blood levels in humans, or whether such levels are the same for all age groups, or the threshold that establishes the need for vitamin D supplementation. This lack of a recommendation is due in part to differences in measurement techniques and the variability of vitamin D levels in different geographical areas. In any case, serum levels of 25(OH)D induced by exposure to sunlight never exceed around 60 ng/mL. It is generally accepted that levels of circulating 25(OH)D of between 30 to 35 ng/dL (75 nmol/L) are adequate for optimal health.98 However, in its most recent recommendations published in 2011, the Institute of Medicine committee cited lower levels.99 After careful review of the evidence, they stated that levels of around 16 ng/mL (40 nmol/L) cover the requirements of approximately half the population, while levels around 20 ng/mL (50 nmol/L) would cover the requirements of 97.5% of the population. The data on the benefits of higher serum levels is scant, particularly with respect to the long-term effects of chronic high concentrations. Serum vitamin D levels of 50 ng/mL (125 nmol/L) should alert the clinician to the possibility of adverse effects.
Environmental Changes, Photoprotection, and Vitamin D: Is There a Dilemma?As discussed above, individual doses of UV radiation depend on many factors. These include both environmental factors (latitude, altitude, aerosols, clouds and dispersion) and social factors (type of work, recreational activities, and cosmetic preferences, among others). An increased awareness in the population about the dangers of solar UV radiation has led to an increase in the use of preventive photoprotective measures, including avoidance of excessive exposure to sunlight and assiduous use of sunscreen. An expert report published by the United Nations Environment Programme (UNEP) outlines a major controversy in the scientific community in that some scientists argue that the adverse effects of UV-B radiation are being overestimated and that its benefits, such as the synthesis and accumulation of vitamin D, are being underestimated.100
In light of this controversy, we must ask ourselves whether the greater use by the population of photoprotective sunscreens to avoid erythema or sunburn in a scenario in which depletion of the ozone layer has slowed down may not lead to a decrease in vitamin D synthesis due to insufficient exposure to sunlight and a consequent loss of its beneficial effects? The 2006 UNEP report on the effects of UV radiation on the environment and human health outlines the debate between 2 groups of scientists on the subject of exposure to sunlight and the balance between its beneficial and harmful effects. One group holds that although UV radiation has a beneficial effect in that it facilitates the synthesis of vitamin D, no simple recommendations can be made to guarantee a balance between the positive effects of vitamin D and the negative effects of overexposure to UV radiation.29 The opposing group argues that the benefits associated with UV-B radiation outweigh the risks.101 This group has even quantified the relationship by estimating that the cost of the vitamin D deficiency in the population as a result of inadequate exposure to sunlight and/or poor diet is between 40 and 56 billion dollars spent on the management of osteoporosis, internal organ cancers, and viral diseases. The same group estimates the cost of excess UV radiation to be between 6 and 7 billion dollars.102
It is, of course, important to be aware that certain population subgroups have a higher risk of vitamin D deficiency, including elderly people who require intensive care or are institutionalized (because they are rarely exposed to sunlight), people with skin cancer or other skin problems who must actively avoid exposure to sunlight, very dark-skinned people, women who wear veils that cover the entire body, and patients with vitamin D malabsorption.
Is Exposure to Sunlight the Primary Source of Vitamin D in the Population?Gilchrest103 demonstrated the relationship between the dose of UV radiation and sunburn, tanning, the formation of DNA photoproducts, and the amount of previtamin D3 in the skin. In the case of sunburn and tanning, the response is dose dependent until a threshold is reached, after which further exposure leads to blistering. In the case of DNA photoproducts, the response increases in a linear fashion from small to very large amounts of UV radiation. In the case of previtamin D3 formation, the amount produced also increases in a linear fashion from small doses of UV radiation until a plateau is reached at a dose lower than 1 minimal erythema dose. This plateau effect occurs because exposure to higher levels of radiation gives rise to the conversion of previtamin D3 into inactive photoproducts, such as lumisterol and tachysterol. Thus, our skin produces previtamin D3 more efficiently than it produces erythema, that is, it synthesizes previtamin D3 with suberythemal doses of UV radiation.
From a more epidemiologic standpoint, it is unclear whether greater exposure to sunlight necessarily produces a larger quantity of vitamin D. Several epidemiological studies of very sunny areas, such as Honolulu, southern Arizona, and southern Florida in the USA and Queensland in Australia, as well as in India, and Brazil found that about 70% of the study population had vitamin D levels below 30 ng/mL.104,105 Consistent with this result, a meta-analysis of 394 studies concluded that latitude does not influence vitamin 25(OH)D levels globally.106 However, in a separate analysis the same authors found a decrease in vitamin 25(OH)D levels with higher latitudes, but only in white-skinned people. However, studies like that of Terushkin et al107 show us how the synthesis of an amount equivalent to 400 IU of vitamin D daily can be obtained through brief exposures to sunlight (3-8minutes) on 25% of the skin surface in white-skinned individuals of different phototypes in a geographical area at the latitude of Miami throughout the year and in Boston from April to October. In other words, the results of this study suggest that latitude is a limiting factor in vitamin D synthesis during the months of October through April at high latitudes. It should not be forgotten that vitamin D levels are not only influenced by cutaneous synthesis, but also by increased destruction of previtamin or vitamin D3, negative regulation of vitamin D synthesis when melanin is synthesized, alterations in vitamin D transport, and probably other factors as yet poorly understood that limit the amount of vitamin D synthesized on exposure to UV radiation. There is no evidence, therefore, allowing us to attribute the vitamin D deficiency observed in populations exclusively to insufficient exposure to sunlight.
A recent study of the effects of artificial UV-B radiation lamps showed that narrowband UV-B phototherapy induces a mean increase in plasma levels of vitamin D in patients with psoriasis of 23 ng/mL to 50 ng/mL.108
Although theoretically sunscreens can almost completely block the production of previtamin D in the skin, this does not occur in practice,109 mainly because of inadequate application of the product to the skin and also possibly due to the fact that individuals who use sunscreen may be exposed to sunlight for more hours than those who do not. Thus, according to Diffey110 and Thieden et al,111 the highest dose of UV radiation is received during the summer months, which is also the period when sunscreens are used more often than any other photoprotective method. The authors of a study of patients with lupus found vitamin D levels to be only weakly related to the use of sunscreens, whereas they found a strong association between such levels and the use of clothing and the practice of limiting exposure to sunlight.112 Therefore, we have no evidence to support the hypothesis that use of sunscreens leads to vitamin D deficiency.
Weinstock and Moses98 concluded that, since there is no evidence that measures taken to prevent skin cancer are the cause of the low vitamin D levels recorded in the world population, abandoning the use of photoprotection measures will not solve the problem. Furthermore, most studies that have demonstrated a benefit associated with an increase in plasma levels of vitamin D have achieved this increase with oral vitamin supplementation. However, dermatologists should bear in mind that cutaneous photosynthesis of vitamin D may be reduced by such measures in many patients to whom we recommend photoprotection, thereby increasing their risk of hypovitaminosis D.
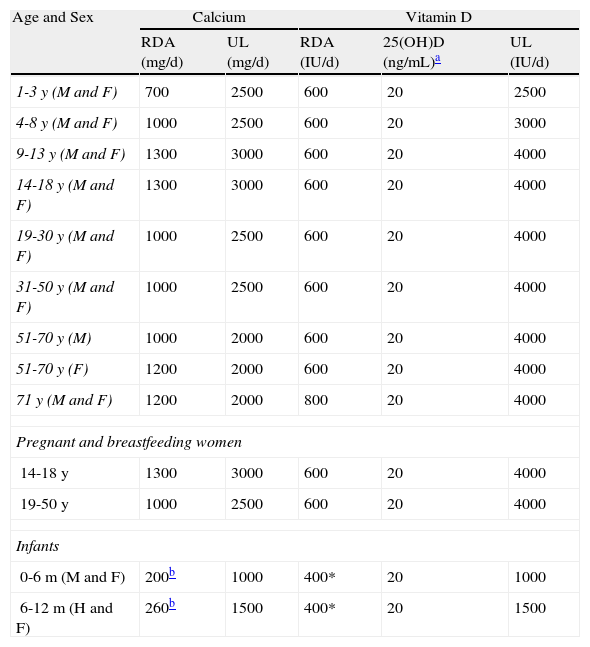
How Can We Prevent and Treat Vitamin D Deficiency?Although factors such as obesity, diet, skin color, and exposure to sunlight, among others, may affect vitamin D levels, only the measurement of vitamin D levels in blood can confirm the existence of a deficiency. Table 5 shows the requirements for calcium and vitamin D calculated by the Institute of Medicine using bone health as an indicator, according to age and sex as well as special circumstances, such as pregnancy and breastfeeding.99 The recommended daily doses for the general population are 600 IU of vitamin D between 1 and 70 years of age and 800 IU above this age; this corresponds to a serum level of 25(OH)D of at least 20 ng/mL (50 nmol/L). When supplements are administered, the recommended doses are 400 or 1000 IU/d of vitamin D with or without calcium, 10 000 IU every 7 or 10 days, or 50 000 IU per month. It is important to monitor levels after treatment because great variability exists between individuals in the increases achieved with these doses.113 If the initial level of 25(OH)D is below 15mg/dL then 50 000 IU should be administered weekly for 8 weeks, after which the dosage should be reduced to the regimen described above. Although vitamin D intoxication has been associated with intake levels of between 50 000 and 1 million IU/d taken for months or even years, the potential risk of kidney stones, vascular damage, and even bone fractures with administration of high doses of vitamin D obliges us to be cautious about indiscriminate administration of vitamin D to the population. Therefore, from a public health standpoint, the only balanced way to improve levels in the population as a whole would be to increase the number of commonly consumed foods fortified with vitamin D.98
Dietary Calcium and Vitamin D Requirements by Age and Sex (American Institute of Medicine).
| Age and Sex | Calcium | Vitamin D | |||
| RDA (mg/d) | UL (mg/d) | RDA (IU/d) | 25(OH)D (ng/mL)a | UL (IU/d) | |
| 1-3 y (M and F) | 700 | 2500 | 600 | 20 | 2500 |
| 4-8 y (M and F) | 1000 | 2500 | 600 | 20 | 3000 |
| 9-13 y (M and F) | 1300 | 3000 | 600 | 20 | 4000 |
| 14-18 y (M and F) | 1300 | 3000 | 600 | 20 | 4000 |
| 19-30 y (M and F) | 1000 | 2500 | 600 | 20 | 4000 |
| 31-50 y (M and F) | 1000 | 2500 | 600 | 20 | 4000 |
| 51-70 y (M) | 1000 | 2000 | 600 | 20 | 4000 |
| 51-70 y (F) | 1200 | 2000 | 600 | 20 | 4000 |
| 71 y (M and F) | 1200 | 2000 | 800 | 20 | 4000 |
| Pregnant and breastfeeding women | |||||
| 14-18 y | 1300 | 3000 | 600 | 20 | 4000 |
| 19-50 y | 1000 | 2500 | 600 | 20 | 4000 |
| Infants | |||||
| 0-6 m (M and F) | 200b | 1000 | 400* | 20 | 1000 |
| 6-12 m (H and F) | 260b | 1500 | 400* | 20 | 1500 |
Abbreviations: F, female; M, male; RDA, recommended dietary allowance; UL, the upper limit above which there is a risk of adverse effects (this limit is not a target value as there is no evidence to suggest that any additional benefit can be obtained by exceeding the recommended daily allowance).
Source: Ross et al.99
The benefits of vitamin D and the best way to achieve and maintain optimal levels are very controversial issues with potentially important implications for human health. Vitamin D enhances musculoskeletal health and reduces mortality related to bone disease in some populations, particularly the elderly and other high-risk groups. Evidence suggests that vitamin D has an impact in cancer, cardiovascular disease, autoimmune processes, and infections. However, while highly suggestive, this evidence is not robust enough to draw definite conclusions or to establish a causal relationship. There are different opinions on what the desirable serum levels of vitamin D may be, and while in recent years 30 ng/mL (75 nmol/L) was considered to be optimal, the most recent Institute of Medicine recommendations indicate that levels of 20 ng/mL (50 nmol/L) appear to be sufficient and achievable for the general population even under conditions of minimal exposure to sunlight. If these figures are reliable, the apparent pandemic of vitamin D deficiency reported in recent years may be exaggerated.
Many of the doubts and controversies concerning the risk-benefit ratio of vitamin D are being researched in a multinational European study entitled ICEPURE: impact of climatic and environmental factors on personal UV radiation exposure and human health (http://www.icepure.eu). We also need a better understanding of the relationship between the maintenance of vitamin D levels through exposure to UVB radiation and through diet and supplementation, and how the 2 sources interact.114
Therefore, more long-term population studies and randomized clinical trials are needed to shed light on the subject and provide evidence that can be used to avoid the problems associated with both deficiency and excess of vitamin D. The most prudent approach at present would appear to be to adopt the guidelines of the Australian and American professional dermatologist's societies, which have warned the public in their countries not to use sunlight as a primary source of vitamin D because of the clear evidence that UV radiation is a skin carcinogen and reiterated that a more healthy approach is to combine limited exposure to sunlight with proper nutrition and supplements when necessary.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gilaberte Y, et al. La vitamina D: evidencias y controversias. Actas Dermosifiliogr.2011;102:572-588.