Onychomycosis, or fungal infection of the nails, is one of the most prevalent fungal diseases in the general population. Treatment is of limited effectiveness, tedious, and must be administered for long periods. Furthermore, systemic antifungal agents are associated with adverse effects. Photodynamic therapy (PDT) may prove to be a viable alternative in the treatment of superficial skin infections, including onychomycosis. We review articles relating to the usefulness of PDT in onychomycosis in both in vitro and in vivo settings and discuss the potential and limitations of various photosensitizing agents. In vivo, methylene blue and 5-aminolevulinic acid have led to cure rates in 80% and 43% of cases, respectively, at 12 months. Finally, based on data in the literature and our own experience, we propose a protocol of 3 PDT sessions, separated by an interval of 1 or 2 weeks, using methyl aminolevulinate 16% as a photosensitizing agent and red light (λ=630nm, 37J.cm−2). Each session is preceded by the topical application of urea 40% over several days. Clinical trials are needed to optimize PDT protocols and to identify those patients who will benefit most from this treatment.
La onicomicosis, o infección fúngica de las uñas, constituye una de las enfermedades micóticas más prevalentes en la población. Su tratamiento tiene una efectividad limitada, además de ser largo y tedioso y, en el caso de los antifúngicos sistémicos, no está exento de efectos adversos. La terapia fotodinámica (TFD) podría ser una buena alternativa para las infecciones cutáneas superficiales, entre ellas la onicomicosis.
El presente artículo revisa la experiencia publicada, tanto in vitro como in vivo, acerca de la utilidad de la TFD en las onicomicosis, mostrando el potencial de diversos fotosensibilizantes, así como sus limitaciones. Desde el punto de vista clínico el azul de metileno y el ácido 5-aminolevulínico muestran tasas de curación del 80% y el 43% respectivamente al año de seguimiento.
Finalmente, basado en la bibliografía y en la propia experiencia, se propone un protocolo de 3 sesiones de TFD, usando metil-aminolevulinato 16% como fotosensibilizante y luz roja (λ=630nm, 37J.cm−2), separadas por 1 o 2 semanas. Estas irán precedidas de la aplicación de urea 40% durante unos días. Nuevos ensayos clínicos deben optimizar los protocolos y establecer qué pacientes se benefician especialmente de recibir este tratamiento.
Onychomycosis is a fungal infection of the toenails or fingernails. It represents up to 50% of all onychopathies, and approximately 30% of all dermatomycosis.1 The reported prevalence ranges from 2% to 40%, depending on the population studied and the diagnostic tools used. Onychomycosis is a common disease in adults2 and is associated with different predisposing factors associated with occupation, social class, age, and climate, as well as a number of underlying diseases, including diabetes, peripheral vascular disease, immune deficiency, and psoriasis.2,3
Dermatophytes are the most common cause of onychomycosis, particularly Trichophyton rubrum, the most common etiologic agent.4–8 Non-dermatophyte filamentous fungi (Fusarium, Aspergillus, Scopulariopsis, and Acremonium species, etc.) cause between 2% and 13% of cases9–12 while yeasts are responsible for about 21% of onychomycosis, usually those affecting the fingernails.8,12
In terms of treatment, both lack of response (40%-70%)2 and relapse or recurrence (20%-25%) are frequent in spite of the progress achieved with new antifungal agents.13 Several factors lead to poor treatment outcomes: the difficulty of achieving penetration of the nail plate, lack of adherence to treatment (which lasts for months), the poor response of some fungi to antifungals, and individual susceptibility.14 There is, therefore, a need to expand treatment options and reduce the adverse effects associated with treatment. Therapies based on devices15 such as laser,16,17 iontophoresis,18 and photodynamic therapy (PDT),19,20 can help overcome the limitations described above.
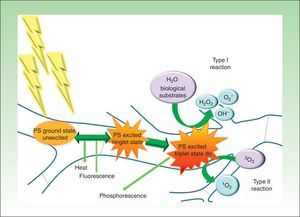
PDT involves the use of photosensitizing agents that selectively localize in certain cells and produce cell death when activated with light of the appropriate wavelength in the presence of oxygen (Fig. 1).21
Modified Jablonski diagram: molecular basis and mechanism of action of photodynamic therapy. The absorption of light by a photosensitizer (PS) in its unexcited ground state promotes an electron to a higher energy orbit (PS excited singlet state). The PS may return to its ground state by emitting heat and/or fluorescence or may change the direction of its angular moment of spin (PS excited triplet state). The long life of the triplet electron state favors the formation of singlet oxygen and/or free radicals that damage key cell structures and eventually cause cell death. At the end of the process, the PS returns to its ground state, ready for a new phototherapeutic cycle.
The use of PDT in the treatment of infections has given rise to antimicrobial PDT, an emerging field of research in the treatment of localized infections.19 Several articles on in vitro and in vivo studies support the usefulness of PDT in the treatment of infections caused by viruses, bacteria, fungi, and parasites.19 In such cases, PDT offers a number of advantages over traditional antimicrobial therapies. These include the following: 1) a broad spectrum of action; 2) effectiveness independent of patterns of antimicrobial resistance; 3) photoinactivation of the microorganisms—a multitarget process that makes the selection of photoresistant strains highly unlikely; 4) availability of formulations that allow specific delivery of the photosensitizer to the infected area and spare adjacent healthy tissue; 5) use of low cost light sources to activate a photosensitizing agent; and 6) compatibility with other antibiotic and antifungal drugs in combination therapies.
The clinical indication of PDT as an antimicrobial has not, however, been approved to date and its application in this setting is anecdotal. In the specific case of fungal infections, mixed results have been reported in a series of cases of ringworm affecting hairless skin22 and of candidiasis.23 Since onychomycosis is a localized infection and because existing treatments are of limited effectiveness, it may be a cutaneous mycosis in which antimicrobial PDT could have a broader application.
Our aim, in this review, is to provide answers to a series of questions about the basic in vitro and clinical in vivo evidence on the use of PDT in onychomycosis.
Is PDT effective in vitro against the filamentous fungi that cause onychomycosis?The antifungal effectiveness of PDT has been assessed, in vitro, in different types of fungi using several different photosensitizers at different concentrations and light sources at various wavelengths. Table 1 summarizes the most important studies.
In Vitro Research into the Use of Photodynamic Therapy in Filamentous Fungi.
| References | Microorganism | Photosensitizer | Light | Effects of PDT | |||
|---|---|---|---|---|---|---|---|
| λ (nm) | Dose (J/cm2) | Fluence (mW/cm2) | Source | ||||
| Morton et al.,31 (2014) | T. rubrum | Rose Bengal | 530 | 24 | 13.4 | Three 3-watt H-HP803PG LED modules | 100% kill at a dose of 140μM |
| Paz-Cristobal et al.30 (2014) | T. rubrumT. mentagrophytes | Hypericin | 602 | 37 | 10.3 | LED | Fungicidal. 3 log reduction at doses of 10-50μM |
| Amorim et al.29 (2012) | T. rubrum | Toluidine blue O | 630 | 18-90 | NS | LED | Fungicidal at a dose of 25μM and an energy density of 72J/cm2 |
| Smijs et al.44 (2009) | T. rubrum(ex vivo human stratum corneum model) | Sylsens B(pH 5.2) | 340-550 | 18 | 30 | UV-A-1 | Fungicidal. Fungal killCMI Sylsens B 10μM with the clinical isolate 1μM laboratory strain |
| Smijs et al.27 (2007) | T. rubrum(ex vivo human stratum corneum model) | Sylsens BDP mme | 580-870 | 108 | 30 | Massive (no. 74900/21). 1×500W-230V-R7s, IP44 with a cut-off filter at 600nm | Fungicidal at different stages of conidial growth.Sylsens B:- 1μM at 8h- 5μM at 17 and 24hDP mme:- 80μM at 8h |
| Donnelly et al.45 (2005) | Trichophyton interdigitale | ALA(0-100mM) | 635 | 100 | 100 | Paterson Lamp (Phototherapeutics Ltd.) | Fungistatic ≤ 79% |
| Kamp et al.28 (2005) | T. rubrum(liquid culture medium) | ALA(1-10mmol l−1) | NS (white light) | 10J for 60min (≈128Jcm−2) | 36.8 | Quartz-halogen lamp Zeiss KL 2500 LCD | Fungistatic: reductions in the number or the diameter of the colonies |
| Smijs et al.26 (2004) | T. rubrum(suspension culture of hyphae and microconidia) | Sylsens BDP mme | 580-870 | 108 | 30 | Massive (no. 74900/21). 13 max. 500 W-230V-R7s, IP44 with a cut-off filter at 600nm | Fungicidal for microconidia:-Sylsens B 1μM-DPmme >5μMFungicidal for hyphae:-Sylsens B 10μM-DPmme 40μM |
| Smijs et al.25 (2003) | T. rubrum(liquid culture medium) | Sylsens BDP mme | NS (white light) | 108 | 30 | Massive (no. 74900/21). 13 max. 500 W-230V-R7s, IP44 | Fungicidal (3μgmL−1) with Sylsens B and DPmme.Fungistatic effect for 1 wk with phthalocyanines and Photofrin |
| Ouf et al.46 (2003) | T. rubrumT. verrucosumT. violaceumMicrosporum canisM. gypseumEpidermophyton floccosum (spore solution) | Hematoporphyrin derivative, methylene blue, toluidine blue | NS (visible light) | 72-144 | 40 | Oriel sun simulator | Fungicidal with hematoporphyrin and methylene blue at 10−3M for M. canis, T. mentagrophytes and T. verrucossum |
| Propst y Lubin24 (1978) | T. mentagrophytesM. gypseum(mixed suspension of spores and mycelia) | Methylene blue,neutral red, proflavine hemisulfate (3mM) | 455 | ≈1.1 | ≈1.8 | Blue light | Fungicidal with proflavine (3mM) |
Abbreviations: ALA, 5-aminolevulinic acid; DPmme, deuteroporphyrin monomethylester; NS, not specified; Sylsens B, 5,10,15-Tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride
In 1978, Propst and Lubin demonstrated that dermatophyte fungi might be photosensitive in vitro to heterocyclic dyes when they observed that proflavine and blue light (455nm) were effective in killing both Trichophyton mentagrophytes and Microsporum gypseum.24
In 2 in vitro studies, Smijs et al.25,26 demonstrated the fungicidal effect of PDT on T. rubrum using porphyrin photosensitizers (Sylsens B and deuteroporfirín monomethylester) activated by broadband red or white light. They observed an effect that persisted several weeks after PDT. Moreover, it was seen at the different stages of fungal growth, although with interesting differences in sensitivity to the PDT: spores in suspension were more susceptible than fungal colonies in liquid culture.26 The fact that PDT destroys the hyphae and inactivates the fungal spores is important in establishing its potential use as a treatment for superficial mycoses. In a clinical situation spores are responsible for the initiation of the infection and often persist and survive in the skin after treatment, favoring reinfection.
The adhesion of dermatophytic fungi to keratinized tissue is essential in the pathogenesis of dermatophytosis. To investigate the outcomes of PDT in a situation similar to a clinical setting, Smijs et al. cultured T. rubrum in an ex vivo model using human stratum corneum.27 Using this model they investigated the susceptibility of the fungus to PDT at different growth phases, demonstrating the importance in fungal virulence of adherence to a keratinized structure. They observed that, compared with the results of in vitro studies, the susceptibility of mature mycelia to PDT decreased while that of conidia did not. PDT using Sylsens B 160μM and red light (108J/cm2) had a fungicidal effect in only 65% of cases, but this figure increased to 90% when a keratinase enzyme inhibitor was added to the incubation mixture. The same authors found that, when photodynamic inhibition was unsuccessful, the photosensitizer had not penetrated the fungal cell wall.
Kamp et al.28 observed a reduction of almost 50% in the growth of T. rubrum in vitro using 5-aminolevulinic acid (ALA) as a photosensitizer. The fungistatic effect could be due to the fact that ALA is a hydrophilic molecule. Consequently, its absorption and metabolism by T. rubrum was extremely slow: the first formation of protoporphyrin IX (PpIX) was observed only after 10 to 14 days of incubation. Increasing the lipophilicity of ALA by esterification is likely to improve its diffusion, uptake, and conversion by T. rubrum.28
Other authors have demonstrated the antifungal action of PDT in vitro using different photosensitizing agents, such as phenothiazines (Amorim et al.),29 hypericin (Peace-Cristobal et al.),30 and Rose Bengal (Morton et al.).31
What Clinical Evidence Supports the Use of Photodynamic Therapy in Onychomycosis?There is little clinical experience with the use of PDT in the treatment of onychomycosis and no standardized protocol exists. Table 2 lists the cases reported and clinical trials published to date. Most of these studies include patients in whom previous antifungal treatments had failed and patients who had underlying diseases that contraindicated oral treatment. All used a light source that emitted a wavelength in the red spectrum, which is not absorbed by hemoglobin and can penetrate more deeply into living tissue, a property that is particularly important in the treatment of nail infections. The lamp most often used in these studies was light emitting diode (LED) with a wavelength of 630±10nm (Aktilite).22,32–35 LEDs are compact, require less energy to emit light at the desired wavelengths, do not cause thermal damage to biological tissues, and are made to produce multiple wavelengths.26
Studies of Onychomycosis Treated with Photodynamic Therapy.
| References | Type of Onychomycosis | Cases No. | Causative Agent | Site | Urea Prior to PDT(%) | Photosensitizer | Incubation Time |
|---|---|---|---|---|---|---|---|
| Watanabe D et al.32 (2008) | Subungual, distal and lateral | 2 | Unspecified DTF | Nail of first toe | Yes, 20% urea | 20% ALA | 5h |
| Sotiriou E et al.22 (2010) | Subungual, distal and lateral | 30 | T. rubrum | Nail of first toe (22 patients)Other toenail (8 patients) | Yes, 20% urea + mechanical abrasion | 20% ALA | 3h |
| Piraccini et al.36 (2008) | Total onychodystrophyProximal subungual | 1 | T. rubrum | Nails on first toes | Yes, 40% urea+ mechanical abrasion | 16% MAL | 3h |
| Aspiroz et al.34 (2011) | White superficial | 1 | Acremonium sclerotigenum | 5th fingernail | None | 16% MAL (Metvix) | 4h |
| Gilaberte et al.35 (2011) | Onychodystrophy | 1 | Fusarium oxysporum | 4th fingernail | Yes, 40% urea | 16% MAL (Metvix) | 4h |
| Gilaberte et al.35 (2011) | White superficial | 1 | Aspergillus terreus | 1st to 5th fingernails | Yes, 40% urea | 16% MAL (Metvix) | 4h |
| Aspiroz et al.33 (2011) | Onychodystrophy. Distal onycholysis | 1 | Candida albicans+Malassezia furfur | 3rd and 4th fingernails | Yes, 40% urea | 16% MAL (Metvix) | 3h |
| Silva et al.39 (2013) | Subungual, distal and lateral. Onychodystrophy | 1 | NS | First toenail both feet both feet | Yes, 20% urea+mechanical abrasion | Hematoporphyrin derivative (Photogem 1mL,mg/mL) | 1h |
| Figueiredo Souza et al.37 (2014) | Subungual, distal and lateral | 40 | T. rubrum, T. mentagrophytesE. floccosumAspergillus nigerCandida sp.Fusarium sp. | NS | Mechanical abrasiona | 2% methylene blue aqueous solution | 3min |
| Figueiredo Souza et al.38 (2014) | Subungual, distal and lateral | 22 | T. rubrum | NS | Mechanical abrasiona | 2% methylene blue aqueous solution |
| References | Follow-up (mo) | Quality of Evidence | No.of PDT Sessions | Light | Rate of Clinical or Microbiological Cure | |||
|---|---|---|---|---|---|---|---|---|
| λ (nm) | Fluence (J/cm2) | Irradiance (mW/cm2) | Source | |||||
| Watanabe D et al.32 (2008) | 3 and 6 | III | 6-7 | 630 | 100 | NS | Pulsed excimer dye laser (Hamamatsu Photonics KK) | 2 (100%) |
| Sotiriou E et al.22 (2010) | 12 and 18 | II | 3 | 570-670 | 40 | 40 | Waldmann PDT 1200 | At 12 months follow-up: 43%At 18 months follow-up: 37% |
| Piraccini et al.36 (2008) | 24 | III | 3 | 630 | 37 | NS | Aktilite | 1 (100%) |
| Aspiroz et al.34 (2011) | 3, 6, 9, and 12 | III | 3 | 630 | 37 | NS | Aktilite | 1 (100%) |
| Gilaberte et al.35 (2011) | 6 | III | 3 | 630 | 37 | NS | Aktilite | 1 (100%) |
| Gilaberte et al.35 (2011) | 6 | III | 3 | 630 | 37 | NS | Aktilite | 1 (100%) |
| Aspiroz et al.33 (2011) | 6 and 18 | III | 3 | 630 | 37 | NS | Aktilite | 1 (100%) |
| Silva et al.39 (2013) | None | III | 6 | 630 | 54 | NS | LED | 1 (100%) |
| Figueiredo Souza et al.37 (2014) | 1 and 12 | II-I | 12 | 630 | 18 | 100 | LED | At the end of treatment: 90%At 12 months follow-up: 80% |
| Figueiredo Souza et al.38 (2014) | 1 and 12 | 12 | 630 | 36 | 100 | LED | Mild to moderate onychomycosis: 100%Severe onychomycosis: 63.3% | |
Abbreviations: ALA, 5-aminolevulinic acid; DTF, dermatophyte fungus; MAL, methyl aminolevulinate; NS, not specified.
Although its effect in vitro is fungistatic, the photosensitizing agent most used in the literature was 20% ALA22,32 or its derivative 16% methyl-aminolevulinate (MAL).33–36 Both have been shown to be effective when applied topically, and have completely disappeared from the treated tissue within 24 to 48hours of application.23 Other photosensitizers used were 2% methylene blue37,38 and a hematoporphyrin derivative (Photogem).39
Most authors report a clinical and microbiological cure rate of 90% to 100% following treatment; however, this percentage decreases on follow-up. The efficacy of PDT appears to depend on the pretreatment of the nail with urea and/or mechanical abrasion to increase its permeability to the photosensitizing agent22,32 and active removal of hyperkeratosis.22,36
The first reported cases of onychomycosis treated with PDT were in 2008, when Watanabe et al.32 treated 2 patients with distal and lateral subungual dermatophyte onychomycosis affecting the first toenail. In both of these patients, other antifungal treatments had been applied with no success. In both cases, the affected nails were pretreated with a 20% urea ointment for 10hours to facilitate penetration of the photosensitizing agent ALA (incubated for 5h). The diseased nails were then irradiated with pulsed laser light at a wavelength of 630nm at 100J/cm2. Treatment was repeated once a week until clinical improvement was observed and no dermatophytes were detected by potassium hydroxide microscopy or by culture. The patients experienced mild pain during PDT, but this disappeared within a day. No recurrence (clinical or microbiological) was observed after 3 months in 1 of the 2 patients or on follow-up at 6 months in the other.
Subsequently, Piraccini et al.36 reported the case of a patient with onychomycosis caused by T. rubrum affecting the first toenails of both feet; topical antifungal treatment had proved unsuccessful and oral therapy was contraindicated. On the days preceding treatment, 40% urea was applied and nail hyperkeratosis was removed. On the day of PDT treatment, 16% MAL was applied and incubated under an occlusive dressing for 3hours. The affected area was then irradiated with red light (Aktilite) (630nm, 37J/cm2). Three PDT sessions separated by an interval of 15 days achieved clinical and mycological cure. No recurrence of the infection was observed on follow-up at 24 months.
Aspiroz et al.33,34 and Gilaberte and colleagues,35 using a protocol based on that of Piraccini et al,36 treated several cases of onychomycosis caused by nondermatophyte molds (Acremonium sclerotigenum, Fusarium oxysporum, Aspergillus terreus) and yeasts (mixed infection with Candida albicans and Malassezia furfur). All these cases of fingernail infections treated successfully with PDT, provide further evidence to support the use of PDT in this type of onychomycosis when the response to oral and topical antifungal therapies is inadequate.40
Silva et al.39 described the effective use of PDT with a hematoporphyrin derivative (Photogem) in the treatment of longstanding onychomycosis. The results of a culture performed after completion of treatment was negative, but there was no subsequent follow-up.
To date, only 3 published clinical trials have been carried out. Sotiriou et al.22 used 20% ALA and red light (40J/cm2, Waldmann PDT 1200) to treat 30 patients with distal and lateral subungual onychomycosis caused by T. rubrum in whom topical antifungal therapy had proved unsuccessful and there were confirmed contraindications to oral antifungal agents. In that study, 22 (73.3%) of the patients had involvement of the first toenail. The number of treatment sessions and the intervals between them were similar to those used by Piraccini et al., with the difference that treatment was preceded by 10 nights with 20% urea under occlusive dressing. On follow-up at 12 months, clinical and microbiological cure was observed in 13 patients (43.3%), no clinical signs were apparent in 5 (16.6%), and in the other 8 (26.6%) patients there were residual changes affecting less than 10% of the nail plate and the results of culture were negative. On follow-up at 18 months, 11 patients (36.6%) were still disease free; the recurrence in 2 cases may have been due to poor penetration of ALA or because only 1 nail was treated when several were infected.
Figueiredo Souza and colleagues37,38 carried out 2 studies using methylene blue as the photosenstizing agent. One of them—the largest trial published to date—enrolled 80 patients with onychomycosis of different etiologies, including T. rubrum, T. mentagrophytes, E. floccosum, Aspergillus spp., Candida spp. and Fusarium spp.37 In this 24-week blind study, patients were randomized to receive either PDT with 2% methylene blue at 15-day intervals or oral fluconazole. In both groups, patients with hyperkeratosis greater than 2mm, longitudinal onychomycosis, or dermatophytomas were treated with mechanical abrasion of the nail to facilitate penetration of the photosensitizing agent. On completion of treatment, the cure rate was 90% in the group treated with PDT and methylene blue versus 45% in the group receiving oral fluconazole (P<.002). After 12 months of follow-up, the cure rate in the PDT group was 80%, irrespective of whether or not the patients had received prior abrasion. The results of that study show that PDT can be an effective treatment for onychomycosis, regardless of the causative pathogen involved. However, the use of oral fluconazole as a comparator may have introduced a bias because this drug is less effective than terbinafine or itraconazole.41,42 The clinical cure rate of 90% is the highest obtained with PDT and is probably due to the use of mechanical abrasion in hyperkeratotic lesions and those with longitudinal spikes or dermatophytoma, features often associated with treatment failure.43
ConclusionsPDT is an easily reproducible, well tolerated, local treatment that does not interact with other drugs and can be combined with any antifungal agent. It is a treatment option for longstanding onychomycosis that has not responded to the usual antifungal therapies and in patients who have an underlying disease, are receiving multiple medications, or do not wish to undertake a prolonged course of treatment.
The variable results obtained in different clinical trials of PDT in onychomycosis may be explained by differences in the causal agent or by factors related to the technical protocol, such as the photosensitizing agent used, the number of sessions administered, pretreatment of the nails, the number of nails affected, and the severity of onychomycosis.
Based on the above data and our own experience over 5 years (Figs. 2 and 3), we believe that PDT can be a good treatment option for patients with onychomycosis of any etiology limited to a few nails when systemic treatment is contraindicated or low adherence to other treatments would be likely. When a subclinical infection is detected or clinical signs of ringworm on the hand or foot are observed, PDT should be complemented by the appropriate treatment.
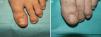
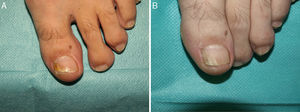
Distal and lateral subungual onychomycosis caused by T. Mentagrophytes. Before (A) and at 36 weeks (B) after treatment with 3 sessions at 1-week intervals of methyl aminolevulinate photodynamic therapy and 16% Aktilite (Photocure ASA, 37J/cm2). Prior to each PDT session the affected area was pretreated with 40% urea under occlusion for 5 consecutive nights.
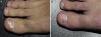
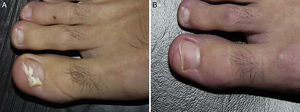
White superficial onychomycosis caused by Fusarium oxysporum. Before (A) and 48 weeks (B) after completion of 3 treatment sessions at 1-week intervals of methyl aminolevulinate photodynamic therapy with 16% Aktilite (Photocure ASA, 37J/cm2). Prior to each PDT sessions the affected area was pretreated with 40% urea under occlusion for 3 nights and active mechanical removal of the nail plate just before the application of the photosensitizing agent.
Table 3 provides details of the PDT protocol for onychomycosis used by our group, which has not been associated with significant adverse effects. An important aspect of this protocol is the pretreatment of the treatment area with 40% urea and mechanical removal of nail residue and hyperkeratotic areas, a procedure that appears to favor the penetration of the photosensitizer, thereby improving the clinical response to PDT. Further clinical trials are needed, not only to establish the real effectiveness of this treatment, but also to optimize the procedure and above all to determine which subgroups of patients may benefit from PDT.
Protocol for Photodynamic Therapy in the Treatment of Onychomycosis.
| Days Before Treatment |
| Apply 40% urea under occlusive dressing 12-24h. Care should be taken not to macerate the periungual skin excessively: |
| In toenails or fingernails with hyperkeratosis>2mm the softening treatment should be applied daily for 5 days prior to photodynamic therapyaIn fingernails or toenails with hyperkeratosis<2mm, 2 or 3 days of softening treatment with urea is sufficienta |
| Day of Treatment |
| 1. Clean the urea residue from the nail plate and surrounding skin using 70% alcohol.2. Remove nail debris and hyperkeratotic mechanically from treatment areas with a scalpel or abrasive tool, such as a file (recommended, could improve the results).3. Clean nail plate and surrounding skin with 70% alcohol.4. Apply the photosensitizer to the nail and periungual area (use a tongue depressor or finger to aid application).5. Cover the whole area with a plastic occlusive dressing and with an opaque dressing to protect area from light for 3h in the case of 16% 5-methyl aminolevulinate acid (Metvix).6. Irradiate with LED 635nm (Aktilite) at a fluence of 37J/cm2.7. Protect the treated area from light for 24h–48h.8. Repeat the procedure every 1-2 weeks for up to 3 sessions.b |
The authors declare no conflict of interest.
The authors would like to acknowledge the help they received from the Spanish Ministry of Economy and Competitiveness for its support of their research in photodynamic therapy (Project CTQ2013-48767-C3-2-R) and the support of the Government of Aragon's B85 Research Group.
Please cite this article as: Robres P, Aspiroz C, Rezusta A, Gilaberte Y. Utilidad de la terapia fotodinámica en el manejo de la onicomicosis. Actas Dermosifiliogr. 2015;106:795–805.