Systematic reviews are one of the most important sources of information for evidence-based medicine. However, there is a general impression that these reviews rarely report results that provide sufficient evidence to change clinical practice.
The aim of this study was to determine the percentage of Cochrane Skin Group reviews reporting results with the potential to guide clinical decision-making.
Material and methodsWe performed a bibliometric analysis of all the systematic reviews published by the Cochrane Skin Group up to 16 August, 2012. We retrieved 55 reviews, which were analyzed and graded independently by 2 investigators into 3 categories: 0 (insufficient evidence to support or reject the use of an intervention), 1 (insufficient evidence to support or reject the use of an intervention but sufficient evidence to support recommendations or suggestions), and 2 (sufficient evidence to support or reject the use of an intervention).
ResultsOur analysis showed that 25.5% (14/55) of the studies did not provide sufficient evidence to support or reject the use of the interventions studied, 45.5% (25/25) provided sufficient but not strong evidence to support recommendations or suggestions, and 29.1% (16/55) provided strong evidence to support or reject the use of 1 or more of the interventions studied.
ConclusionsMost of the systematic reviews published by the Cochrane Skin Group provide useful information to improve clinical practice. Clinicians should read these reviews and reconsider their current practice.
Las revisiones sistemáticas son una de las fuentes más importantes de Medicina basada en la evidencia. No obstante, existe una impresión de que estas revisiones rara vez aportan resultados con evidencia suficiente para cambiar nuestra práctica.
El objetivo de este trabajo es determinar el porcentaje de revisiones publicadas por el Cochrane Skin Group (Grupo Cochrane de Piel) con resultados útiles para guiar nuestras decisiones clínicas.
Material y métodosSe ha realizado un análisis bibliométrico de las revisiones sistemáticas realizadas por el Cochrane Skin Group y publicadas hasta el 16 de agosto de 2012. Se obtuvieron un total de 55 revisiones, las cuales fueron analizadas y clasificadas de forma independiente por 2 investigadores en: 0) no existe evidencia suficiente para apoyar o rechazar ninguna intervención; 1) no existe evidencia suficiente para rechazar o apoyar una intervención pero sí existe suficiente evidencia para hacer recomendaciones o sugerencias; y 2) existe una fuerte evidencia para apoyar o rechazar una intervención.
ResultadosDel total de las revisiones publicadas por el Cochrane Skin Group el 25,5% (14/55) no mostraban evidencia suficiente en ninguna de las intervenciones estudiadas para sustentar su rechazo o aprobación. Un 29,1% (16/55) obtuvo resultados con una fuerte evidencia a favor o en contra de alguna de las intervenciones estudiadas y el 45,5% (25/55) mostraba evidencia suficiente, aunque no fuerte, para hacer sugerencias o recomendaciones.
ConclusionesLa mayoría de las revisiones sistemáticas del Cochrane Skin Group aportan información útil para mejorar nuestra actividad clínica. Los clínicos deberían leerlas y compararlas con su práctica actual.
Evidence-based medicine is an approach to health care in which the clinician's decisions are taken on the basis of the available scientific evidence with a view to improving patient care.1
Given the large quantity of biomedical information now available, it is difficult for practitioners to keep abreast of the medical literature and to identify the most relevant and best evidence for each topic. Thus we need tools, such as systematic reviews, that facilitate this process by evaluating the data and summarizing the strength of the available evidence, which is rated according to study type.
In the biomedical evidence resource pyramid, systematic reviews are rated as the best source of information. Their preparation requires a structured approach and a critical analysis to eliminate or minimize possible bias and random error.2 The first step in the preparation of a systematic review is a comprehensive search of all existing studies in the literature on the specific topic. Explicit inclusion criteria based on strength of evidence are used to select the studies. The strength of evidence provided varies according to study design. Once the quality of the studies identified has been assessed, the authors summarize the results obtained (whether they are qualitative, quantitative, and/or meta-analyses) and interpret the findings and their implications for clinical practice and research.
The number of published systematic reviews has increased considerably, mainly because of the work of the Cochrane Collaboration, an international network formed in 1993 to prepare and disseminate high-quality systematic reviews that examine the effects of health care interventions.3,4 Cochrane reviews, published in the Cochrane Database of Systematic Reviews, are the product of working groups of volunteers from all over the world. The systematic reviews these groups prepare are of high quality because they follow rigorous protocols and methods of review and evaluation that have been designed to minimize error and bias. As a result, the Cochrane database is one of the most reliable sources of biomedical evidence available.2,5,6
Until recently, there were relatively few systematic reviews in the field of dermatology compared to other medical specialties.7 This situation is changing, thanks in part to the work of the Cochrane Skin Group.8,9 Since its creation in 1997, this group has produced systematic reviews for a wide range of dermatologic interventions. Even though systematic reviews are a key tool in evidence-based dermatology, there remains a subjective notion that the findings of such reviews often fail to provide sufficient evidence to guide our clinical practice.
The aim of this study was to determine the percentage of Cochrane Skin Group systematic reviews that report results that are useful to the clinician.
Materials and MethodsWe performed a literature search to identify all the completed reviews published by the Cochrane Skin Group between its launch in 1997 and August 16, 2012.10
No inclusion or exclusion criteria were applied; that is, all the group's published reviews were evaluated.
In every case, the full text was retrieved together with the full title and all author details, the year of publication, and the authors’ conclusions.
All the reviews were evaluated independently by 2 researchers, who classified them according to their usefulness in clinical practice into the following categories:
- -
Not useful in clinical practice: insufficient evidence to support or reject the use of an intervention.
- -
Useful: insufficient evidence to support or reject the use of an intervention, but sufficient evidence to support recommendations or suggestions.
- -
Very useful: strong evidence to support or reject the use of an intervention.
- -
Unclassifiable.
The 2 reviewers (PDS and AB) independently collected the information and recorded it in Microsoft Excel 2007.
When the 2 principal reviewers did not agree on the classification of an article, the review in question was evaluated by a third investigator (IGD).
ResultsAll 55 systematic reviews identified by the search strategy were analyzed in their entirety (Tables 1 and 2).
List of Cochrane Skin Group Systematic Reviews Considered Very Useful in Clinical Practice (Strong Evidence to Support Recommendations Concerning One or More of the Therapeutic Interventions Reviewed) Together With a Summary of the Authors’ Recommendations.
| Title | Recommendations |
| Interventions for bullous pemphigoid | Starting dosages of prednisolone higher than 0.75mg/kg/d do not offer any additional benefit. Lower doses (0.5mg/kg/d) may be sufficient to control disease in most patients. The low-dose regimen could reduce the incidence and severity of the adverse effects associated with treatment (especially mortality).Very potent topical corticosteroids (for example, clobetasol propionate) are effective and appear to have fewer adverse effects than high-dose regimens of systemic corticosteroids; however, their use in extensive disease may be limited by practical factors relating to their application. They should be considered as the first-line therapy whenever possible, particularly when disease is localized. The use of large quantities can lead to systemic absorption of the corticosteroid and adverse events. |
| Interventions for chronic palmoplantar pustulosis | The efficacy of topical corticosteroids increases when hydrocolloid dressings are used to occlude the treated area: clearing was achieved in 2 out of 3 patients within 12 days. Oral PUVA can induce clearance in 2 out of 5 patients. Systemic retinoids at 0.5mg/kg/d produced improvement in 2 out of 3 patients and a good to excellent response in 2 out of 5. Maintenance therapy with retinoids reduces the incidence of recurrence. Combining retinoid therapy with PUVA appears to enhance the efficacy of both therapies and induce clearing in 2 out of 3 patients. There is evidence that tetracycline antibiotics and ciclosporin can be beneficial in the treatment of palmoplantar pustulosis |
| Interventions for female pattern hair loss | Evidence supports the efficacy and safety of topical minoxidil in the treatment of female pattern hair loss. Studies are needed to compare the application of minoxidil5% once a day to minoxidil2% twice daily. |
| Interventions for the skin infection impetigo | Good evidence suggests that topical mupirocin and topical fusidic acid are equally, or more, effective than oral treatment in patients with limited disease. Both have shown similar efficacy. It is unclear whether oral antibiotic therapy is superior to topical antibiotics in patients with extensive disease. Penicillin was found to be less effective than other oral antibiotics. The pattern of antibiotic resistance should be taken into account when choosing appropriate antibiotic therapy. |
| Interventions for ingrowing toenails | Surgical interventions are more effective than nonsurgical treatments in preventing the recurrence of an ingrowing toenail. Surgical intervention in combination with nail matrix phenolization appears to prevent recurrence more effectively than surgery alone. No evidence is available to support the use of the postoperative administration of prophylactic antibiotics to reduce the risk of complications. |
| Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita | Benign mucous membrane pemphigoid with mild to moderate activity responds to dapsone in most patients. Dapsone should therefore be the first-line treatment, given that it is less toxic than cyclophosphamide. There is moderate evidence for a response to cyclophosphamide combined with topical steroids |
| Interventions for photodamaged skin | Clear evidence shows that topical tretinoin improves the appearance of mild to moderate photodamage on the face and forearms in the short term. However, scaling, dryness, irritation, and burning may initially be experienced. |
| Interventions for rosacea | Topical metronidazole, azelaic acid, and anti-inflammatory doses of doxycycline (40mg) appear to be safe and effective for papulopustular rosacea in the short term. There is evidence that a doxycycline dosage of 40mg/d is as effective as 100mg/d and that the lower dose is associated with fewer adverse effects. |
| Interventions for toxic epidermal necrolysis | Treatment with thalidomide has not been shown to be effective and has been associated with higher mortality in placebo-controlled trials. There are no randomized clinical trials in this setting assessing the efficacy and safety of other interventions, such as systemic corticosteroids, ciclosporin, and immunoglobulins. |
| Lasers or light sources for treating port-wine stains | Pulsed dye laser treatment leads to clinically relevant clearance of port-wine stains |
| Oral treatments for fungal infections of the skin of the foot | Terbinafine appears to be more effective than griseofulvin in the treatment of tinea pedis. Terbinafine and itraconazole are more effective than placebo. Terbinafine (2 wks) is more effective than itraconazole (2 wks). No significant differences were found between terbinafine (2 wks) and itraconazole (4 wks), between fluconazole and either ketoconazole or itraconazole, between griseofulvin and ketoconazole, or between different doses of fluconazole. |
| Systemic antifungal therapy for tinea capitis in children | Terbinafine, fluconazole, and itraconazole are as effective as griseofulvin in the treatment of tinea capitis caused by Trichophyton species in children. Shorter treatment durations of these antifungals may improve adherence; the safety profile of such short regimens is good in children. |
| Topical pimecrolimus for eczema | Topical pimecrolimus is less effective than moderate and potent topical corticosteroids and tacrolimus 0.1%. |
| Topical treatments for chronic plaque psoriasis | Evidence suggests that vitamin D analogs are more effective than the emollient alone. Potent and very potent corticosteroids are also effective and very potent corticosteroids are more effective than either potent corticosteroids or vitamin D analogs. The effectiveness of dithranol and tazarotene appears to be similar to that of vitamin D analogs. Corticosteroids appear to be more effective than vitamin D in psoriasis of the scalp, whereas these treatments are equally effective for psoriasis on the rest of the body. Combined treatment with vitamin D and corticosteroids is more effective than either treatment in monotherapy. Vitamin D is more effective than coal tar, but the results on the relative effectiveness of vitamin D and dithranol were mixed. Occlusion improves the effectiveness of vitamin D analogs if the application is done twice daily rather than once a day. |
| Topical treatments for cutaneous warts | There is clear evidence that topical formulations containing salicylic acid are effective. There is less evidence supporting cryotherapy, a treatment that does not appear to offer better results than simpler and safer methods. |
| Topical treatments for fungal infections of the skin and nails of the foot | Topical allylamines and azoles produce much higher cure rates than placebo in athlete's foot. Allylamines were associated with a slightly higher cure rate than azoles. |
Abbreviations: PUVA, psoralen UV-A phototherapy.
List of Cochrane Skin Group Systematic Reviews Not Included in Table 1 (Not Classified as “Very Useful”.
| Title |
| Reviews classified as “useful in clinical practice” |
| Chinese herbal medicine for atopic eczema |
| Dietary exclusion for established atopic eczema |
| Drugs for discoid lupus erythematosus |
| Interventions for American cutaneous and mucocutaneous leishmaniasis |
| Interventions for Old World cutaneous leishmaniasis |
| Interventions for basal cell carcinoma of the skin |
| Interventions for cellulitis and erysipelas |
| Interventions for erosive lichen planus affecting muscosal sites |
| Interventions for erythema nodosum leprosum |
| Interventions for infantile hemangiomas (strawberry birthmarks) of the skin |
| Interventions for melasma |
| Interventions for pemphigus vulgaris and pemphigus foliaceus |
| Interventions for pityriasis rosea |
| Interventions for preventing non-melanoma skin cancers in high-risk groups |
| Interventions for preventing occupational irritant hand dermatitis |
| Interventions for skin changes caused by nerve damage in leprosy |
| Interventions for vitiligo |
| Interventions to reduce Staphylococcus aureus in the management of atopic dermatitis |
| Laser and photoepilation for unwanted hair growth |
| Probiotics for treating eczema |
| Psychological and educational interventions for atopic eczema in children |
| Safety of topical corticosteroids in pregnancy |
| Systemic treatments for metastatic cutaneous melanoma |
| Topical interventions for genital lichen sclerosus |
| Reviews classified as “not useful in clinical practice” |
| Antistreptococcal interventions for guttate and chronic plaque psoriasis |
| Chemoimmunotherapy versus chemotherapy in metastatic malignant melanoma |
| Disposable nappies for preventing napkin dermatitis in children |
| Histamine H2-receptor antagonists for urticaria |
| Interventions for alopecia areata |
| Interventions for cutaneous molluscum contagiosum |
| Interventions for guttate psoriasis |
| Interventions for non-metastatic squamous cell carcinoma of the skin |
| Laser resurfacing for facial acne scars |
| Minocycline for acne vulgaris: efficacy and safety |
| Oral potassium iodide for the treatment of sporotrichosis |
| Statins and fibrates for preventing melanoma |
| Surgical excision margins for primary cutaneous melanoma |
| Topical vitamin A, or its derivatives, for treating and preventing napkin dermatitis in infants |
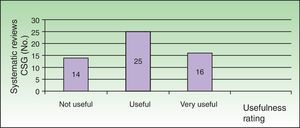
In 14 of the reviews (25.5%), the evidence was insufficient to support the rejection or recommendation of any of the interventions studied (Fig. 1).
Classification of Cochrane Skin Group systematic reviews according to clinical usefulness: not useful for clinical practice (insufficient evidence to support any recommendation for or against the interventions studied); useful (the evidence found is not strong but is sufficient to make recommendations or suggestions on some of the interventions studied); and very useful (strong evidence was found for or against at least 1 of the interventions studied).
In 16 (29.1%), the results obtained provided strong evidence to support or reject the use of at least 1 of the interventions studied (Fig. 1).
Finally, 25 of the 55 reviews (45.5%) provided moderate evidence (Fig. 1), sufficient to support recommendations or suggestions; these articles also highlighted the lack of strong evidence and the need for good quality randomized clinical trials to remedy this deficit.
The 2 principal reviewers did not initially agree on the classification of 9 of the 55 systematic reviews. These discrepancies were resolved when they reassessed these reviews together, and no further assessment on the part of the third reviewer was needed.
DiscussionDermatology has taken longer than other specialties to incorporate evidence-based medical practice. This delayed adoption is reflected in the smaller number of high-quality clinical trials that have been undertaken to assess dermatologic therapies as compared to other specialties such as cardiology, rheumatology, and internal medicine.1,11,12
The efficacy of many of the therapies referred to in dermatology as “classic” has never been proven in randomized clinical trials, and most of them have never been tested in placebo-controlled trials. Possibly, dermatologists’ acceptance of empirical therapies is a result of the belief that better evidence is unavailable. This assertion should not, however, be made until at least one high-quality systematic review has been performed.
The more information we have at our disposal, the more we need tools, such as systematic reviews, that synthesize the information and reduce our need to access multiple primary sources.12
In this study, we analyzed all the reviews published by the Cochrane Skin Group before August 2012. The results obtained demonstrate the practical usefulness of these reviews since over 70% provide useful evidence concerning the use of therapeutic and preventive interventions in dermatology. Only 26% of these reviews did not provide sufficient evidence to support any recommendations. Although a systematic review that does not find sufficient evidence is of little use to the clinician, it is still of use to the specialty because it underscores the need for quality research to address a knowledge deficit and stimulates further research in the area in question.
One of the strengths of our study is the high level of reproducibility of the method used to classify the studies, which generated few doubts. Another strength is that we only evaluated systematic reviews published by the Cochrane Skin Group, thereby ensuring the quality of the review articles and the reliability of the recommendations.
However, a limitation is our exclusive focus on the reviews by the Cochrane Skin Group rather than including other, increasingly numerous, sources of systematic reviews that may be relevant to dermatologic practice; among candidates for inclusion would have been systematic reviews produced by other working groups in the Cochrane Collaboration. It should also be remembered that systematic reviews are updated periodically and that conclusions may change over time.
Our findings demonstrate an improvement over the results obtained by Parker et al.7 in 2001. Those authors analyzed the systematic reviews on dermatologic issues in the Database of Abstracts of Reviews of Effectiveness (DARE) and The Cochrane Collaboration. We note that while the selection and classification methods used in that study and ours were slightly different, we found a significantly higher percentage of reviews that provided sufficient evidence to support recommendations (74.5%) than Parker et al did. (40%). This discrepancy could be explained by differences in design, the growing number of high-quality randomized trials being carried out in dermatology, or by the choice of systematic review topics more oriented to fields with sufficient evidence.
ConclusionsThe present study demonstrates the practical use of the systematic reviews published by the Cochrane Skin Group. Three fourths of these reviews provided sufficient evidence to make some kind of recommendation or suggestion in favor or against a particular intervention. These recommendations could serve to improve our daily clinical practice, help us in our decisions, and lead us to question certain commonly established practices. Systematic reviews that do not provide enough evidence to support useful recommendations are also useful in that they guide future research.
Comparing our practice with the recommendations of the Cochrane Skin Group reviews is a good way to improve the care we give our patients.
Ethical DisclosuresProtection of human and animal subjectsThe authors state that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace concerning the publication of patient data, and that all the patients included in this study were appropriately informed and gave their written consent to participate in this study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Davila-Seijo P, et al. Utilidad de las revisiones del Cochrane Skin Group para la práctica clínica. Actas Dermosifiliogr. 2013;104:679–84.