Advances in our understanding of the biology and therapy of vascular anomalies have made this condition a common reason for consulting a dermatologist. In addition, multidisciplinary units have been created to manage patients with complex vascular anomalies. Although most vascular anomalies are diagnosed based on clinical findings, a thorough evaluation often requires additional imaging tests to determine the nature, extension, and prognosis of these lesions. Because it is fast and noninvasive, ultrasound is usually the first imaging test ordered. In the present review, we provide a state-of-the-art synthesis of key concepts in the ultrasound examination of vascular anomalies so that they are more accessible to clinicians and medical imaging specialists involved in the management of these lesions.
El avance en el conocimiento de la biología y la terapéutica de las anomalías vasculares (AV) han hecho que sean un motivo frecuente de consulta en las consultas de dermatología en la actualidad, y que se hayan creado unidades multidisciplinares para el abordaje de los pacientes con AV complejas.
Aunque el diagnóstico de la mayoría de las AV es clínico, a menudo su estudio completo requiere pruebas complementarias de imagen para determinar su naturaleza, extensión y pronóstico. La primera prueba de imagen que se solicita por su rapidez e inocuidad es la ecografía (US).
En esta revisión se busca resumir y actualizar los conceptos clave en la ecografía de las AV para su mejor comprensión para los clínicos o especialistas en imagen que tratan a estos pacientes.
Since the publication in 1999 by Dubois et al.1 of the ultrasound characteristics of vascular soft tissue tumors, rapid progress has been made in this field of dermatology. Examples of this progress include:
- 1)
Definitive adoption of the classification of vascular anomalies (VAs) published by the International Society for the Study of Vascular Anomalies and updated yearly2
- 2)
Description of the mutations in syndromes that accompany these VAs3
- 3)
Generalized use of propranolol as first-line treatment of hemangiomas4
- 4)
Advances in vascular treatments based on lasers and other light sources5
- 5)
The creation of multidisciplinary units for the integrated treatment of these anomalies6
Ultrasound imaging is usually the first diagnostic test performed on patients with VA given that it is harmless, quick, and readily available in many centers both in primary care and specialist care.7
In view of the above, the multidisciplinary teams that attend these patients should have a clear understanding of the concepts used in ultrasound imaging of VAs, and the applications and limitations of this technique.
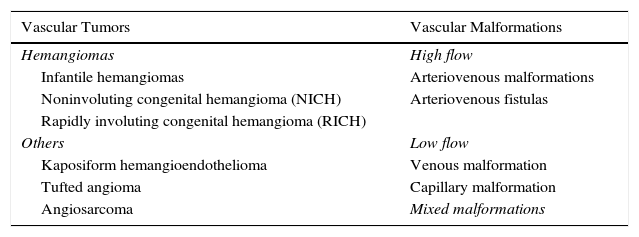
General Considerations in Ultrasound Imaging of Vascular AnomaliesFrom the hemodynamic point of view, VAs are classified as high- or low-flow anomalies.8,9
This distinction is important from the diagnostic and therapeutic point of view, as a preference for pharmacological treatment, surgery, different types of laser therapy, or sclerotherapy, will depend on the hemodynamic characteristics of the lesion (Table 1).
Modified Mulliken-Glowacki Classification.
| Vascular Tumors | Vascular Malformations |
|---|---|
| Hemangiomas | High flow |
| Infantile hemangiomas | Arteriovenous malformations |
| Noninvoluting congenital hemangioma (NICH) | Arteriovenous fistulas |
| Rapidly involuting congenital hemangioma (RICH) | |
| Others | Low flow |
| Kaposiform hemangioendothelioma | Venous malformation |
| Tufted angioma | Capillary malformation |
| Angiosarcoma | Mixed malformations |
In ultrasound imaging of VAs, B mode (grey scale) and Doppler characterization are essential.
B mode imaging is used to define the lesion profile; for example, lesions with a solid appearance in ultrasound imaging are usually vascular tumors, whereas malformations consist of elements with a sponge-like appearance.
Doppler imaging of VAs should always be done in color or power Doppler mode, as well as in pulsed or spectral Doppler mode.
Color Doppler imaging provides information on the presence of blood flow. Pulsed Doppler imaging reveals information on the hemodynamic characteristics of the vessels of the anomaly.
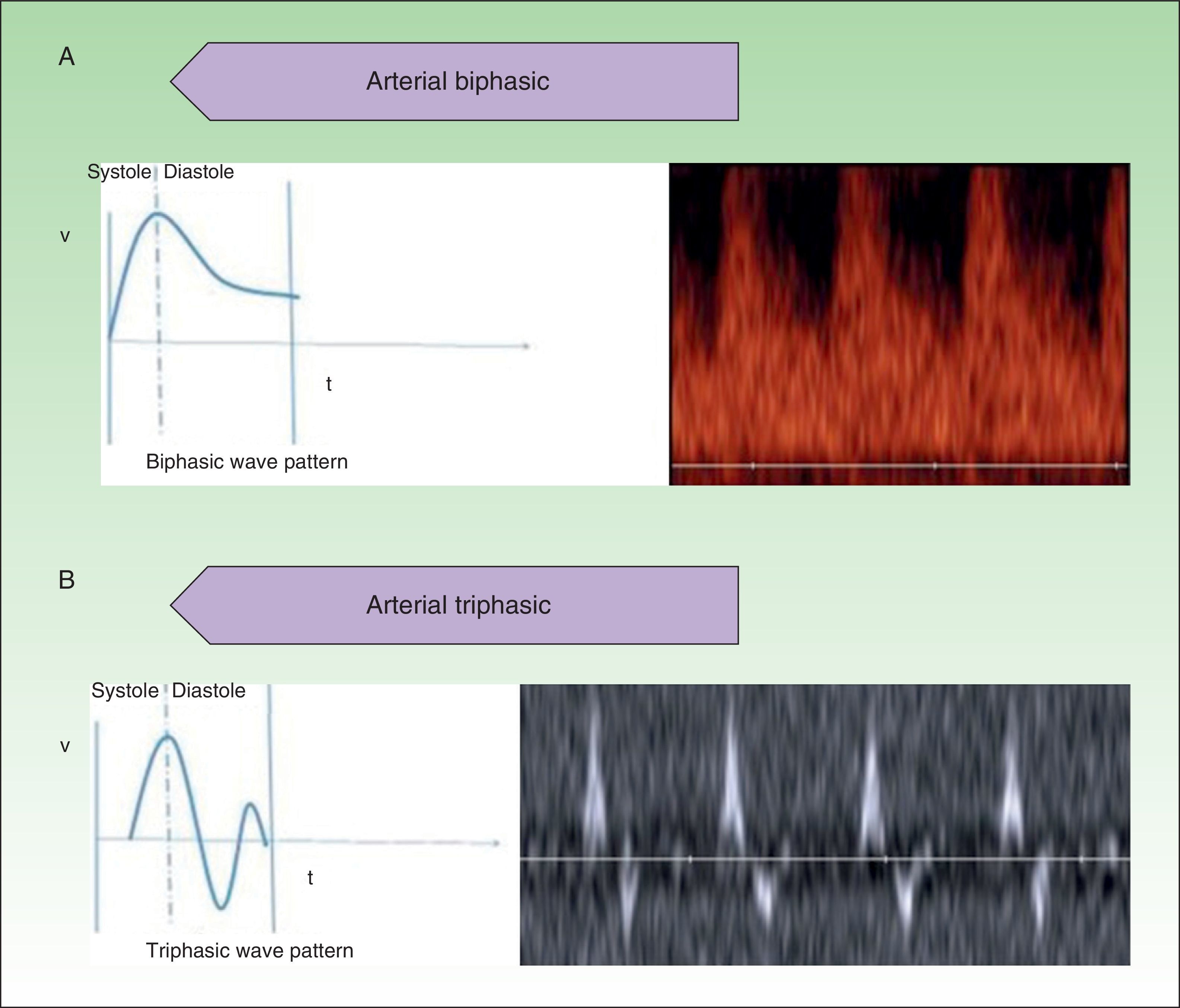
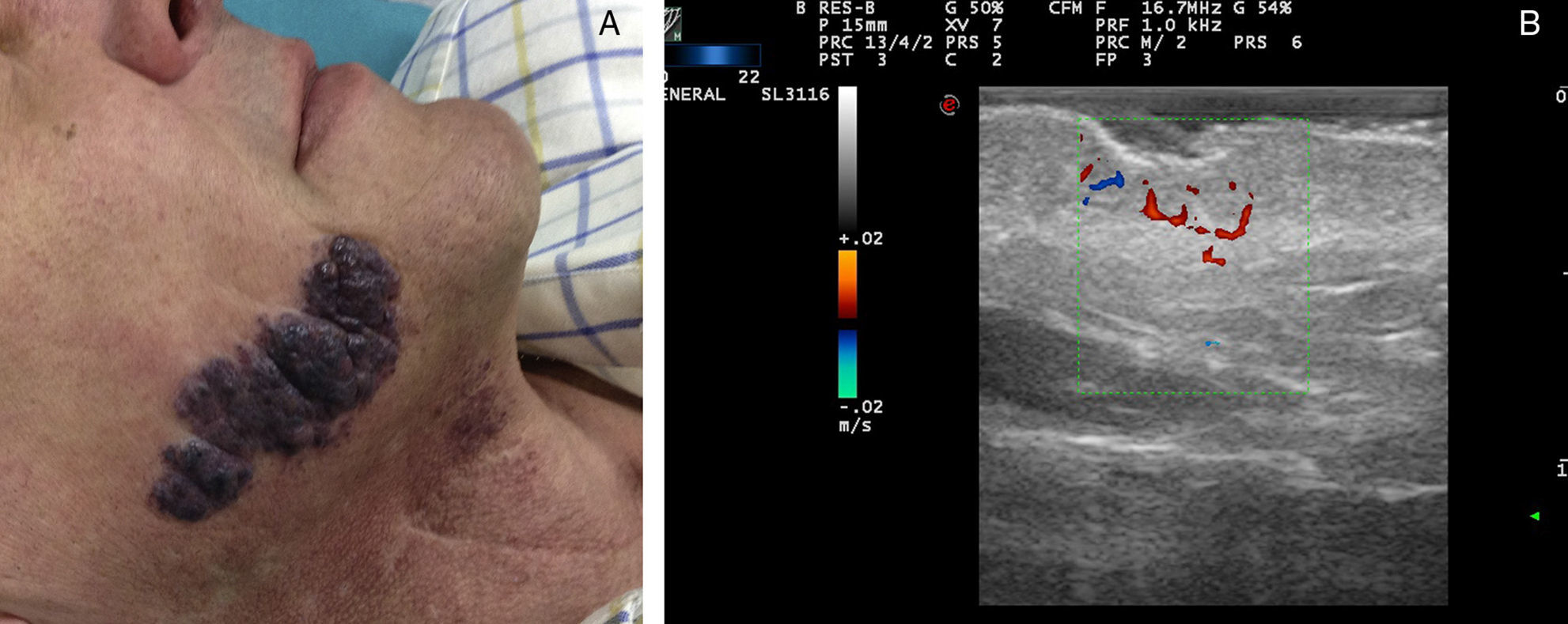
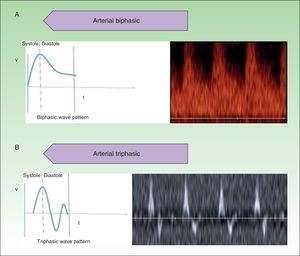
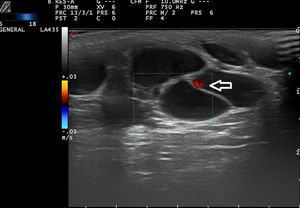
High-flow VAs are defined as those anomalies with an arterial Doppler spectrum, whether of high or low resistance (Fig. 1).
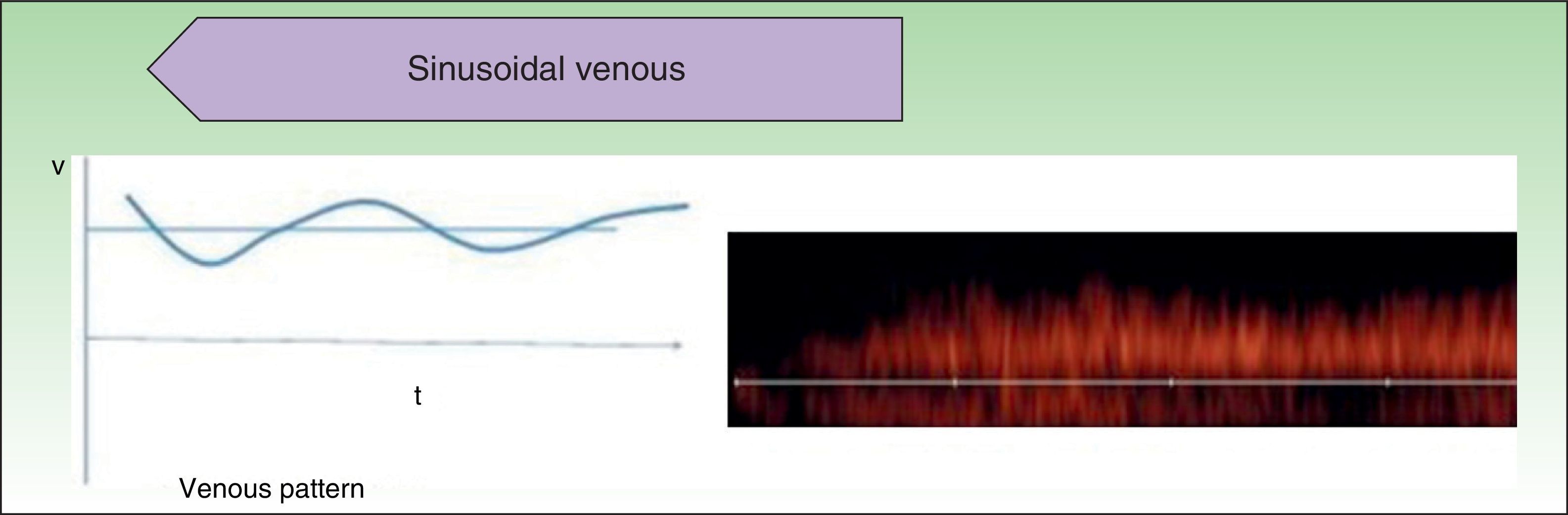
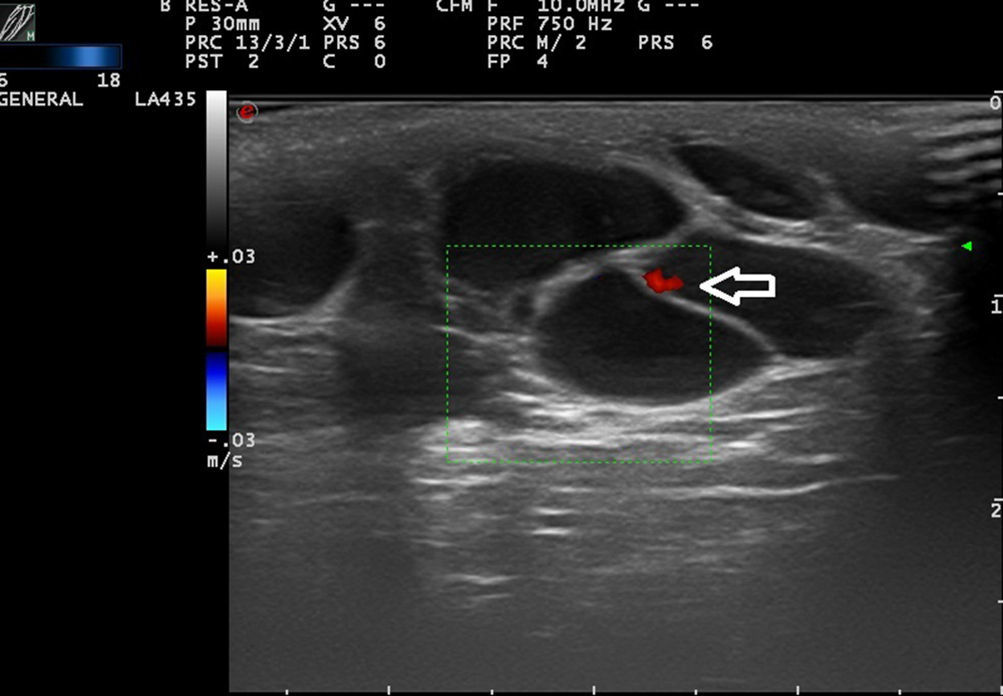
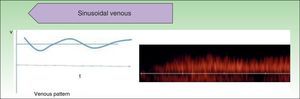
Low-flow VAs correspond to anomalies in which we find phasic flow patterns (as in venous vessels) or the absence of flow (Fig. 2).
At least 3 flow cycles the peak systolic velocity in the lesions and fully characterize them.9
The hemodynamic parameter most frequently assessed in vascular lesions is the resistance index (RI), which is the difference between peak systolic velocity and end-diastolic velocity divided by peak systolic velocity.10 This parameter provides information on the vascular resistance in the vascular bed being studied.
HemangiomasInfantile HemangiomasInfantile hemangiomas are the most common vascular tumors in children, with an incidence of between 4% and 10%.11 They occur more frequently in white girls and most are sporadic. In general, they are not present at birth and progress in 3 characteristic phases. The first is a proliferative phase that lasts for the first 6 months of life, followed by a stabilization phase during the first year. Finally, there is an involuting phase that lasts until 5 to 7 years of age.
These are benign lesions with a favorable outcome and most do not require treatment. However, in 10% of cases, complications may occur, such as ulceration and life-threatening functional alterations, for example airway hemangiomas.
In these cases, propranolol, a nonselective β-blocker, has become the treatment of choice.12 The mechanisms of action that have been proposed include vasoconstriction, angiogenesis inhibition, and endothelial cell apoptosis.13
In general, diagnosis of infantile hemangiomas is clinical, but occasionally, due to the site or involvement of vital structures, complementary studies such as ultrasound imaging, computed tomography (CT), or magnetic resonance imaging (MRI) are required.14
Both CT and MRI generate anatomical information such as exact delimitation of the borders and size and determination of the proximity to adjacent structures. However, these techniques have certain drawbacks such as the need for sedation in children or the need for contrast agents that emit radiation, not to mention the high cost. CT is a reliable method for assessing bone and erosive structures. MRI is considered the method of choice for the study of cervicofacial hemangiomas to rule out posterior fossa malformations–hemangiomas–arterial anomalies–cardiac defects–eye abnormalities–sternal cleft and supraumbilical raphe syndrome (commonly known as PHACES syndrome).15
Ultrasound imaging is a noninvasive method that can be performed in an outpatient setting. The study provides immediate results and sedation is not required, although some authors are including sedation with chloral hydrate in their daily practice to reduce artifacts when performing the Doppler study.16,17 Spierer et al.18 established diagnosis in 20 patients with periorbital infantile hemangiomas without resorting to other imaging studies. The authors considered the technique the method of choice for detecting infantile hemangiomas in children.
Ultrasound Imaging in Infantile HemangiomasUltrasound imaging is currently the method of choice for assessing infantile hemangiomas because the technique allows measurement of the size, thickness, internal characteristics, and vascularization of the lesion, while involvement of adjacent anatomical structures can also be assessed.18
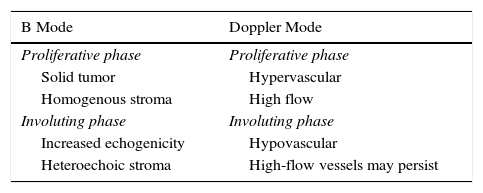
Infantile hemangiomas have different ultrasound patterns (Table 2), depending on the clinical phase. In the proliferative phase, in B mode, a well-defined solid, hypoechoic tumor mass is observed with a lobulated, homogeneous stroma. The color Doppler image is characterized by hypervascular lesions, with a vessel density>5 vessels/cm2 and a systolic Doppler shift >2kHz and a low resistive index (RI).19
In the involuting phase, however, the hypervascularized stroma of the proliferative phase is replaced by fibroadipose tissue, thereby changing the echogenicity, with most lesions now being hyperechoic and heterogenous. In most cases, there is a decrease in color Doppler flow, but high systolic flow may persist compared with normal skin.
Treatment with propranolol is effective in all growth phases of infantile hemangioma, but it is more effective if treatment is started during the proliferative phase. Bingham et al.20 reported the effectiveness of propranolol in 24 patients. At the end of treatment, a decrease in the size of infantile hemangioma was observed in 70% when treatment was given in the proliferative phase, whereas the reduction was 30% when treatment was given in the involuting phase.
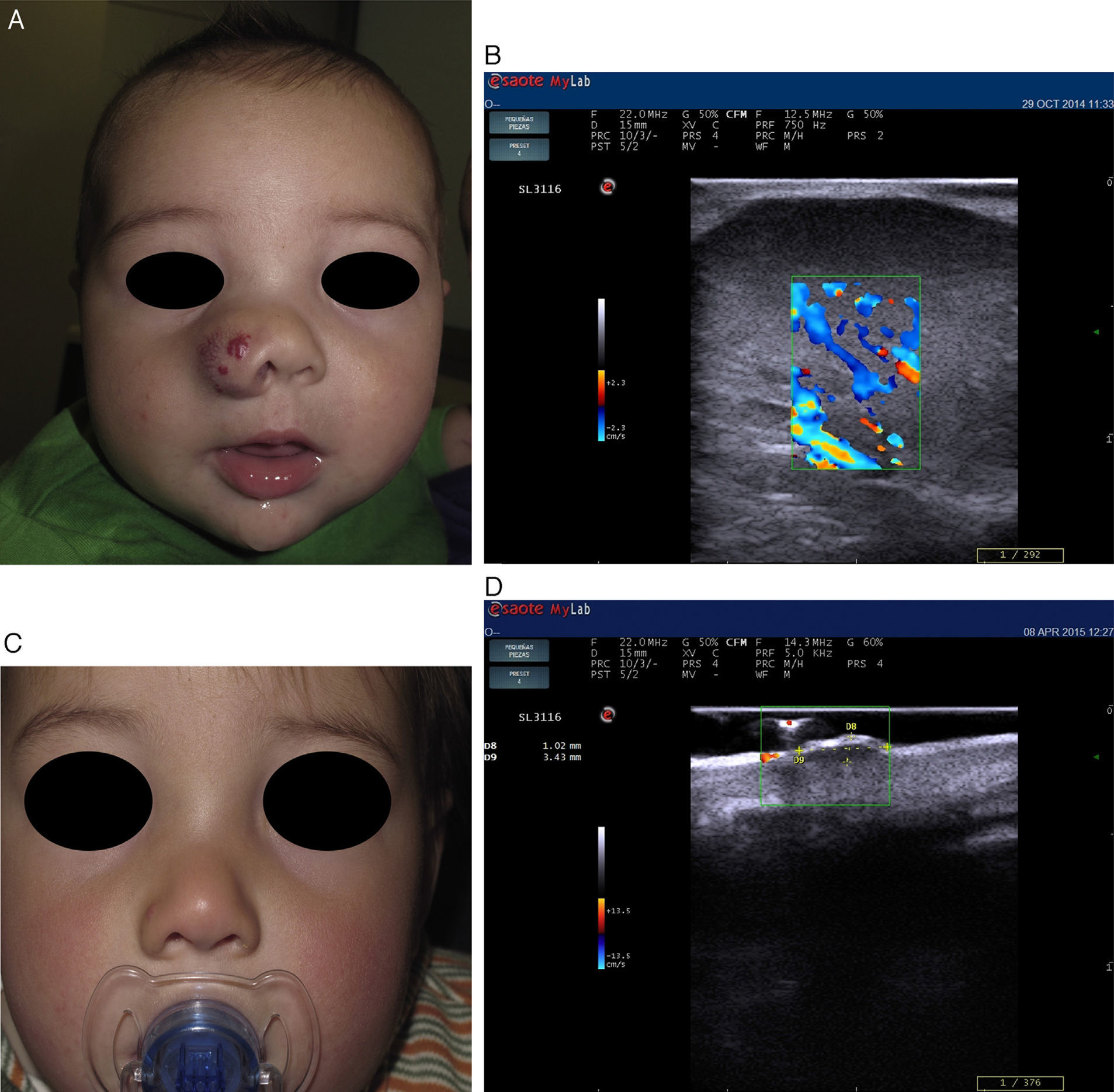
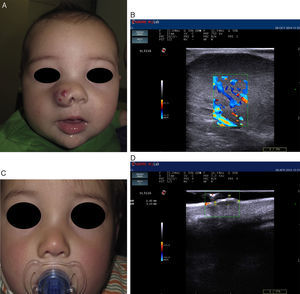
Recent publications describe cutaneous ultrasound imaging as the method of choice for the objective assessment of treatment efficacy with propranolol in complex cases of infantile hemangioma (Fig. 3).21 Sans et al.19 used ultrasound imaging to assess the maximum thickness of infantile hemangioma and RI at baseline and after 60 days of treatment. They observed a decrease in the thickness associated with increased RI.21,22
Infantile hemangioma on the right nostril treated with propanolol (courtesy of Dr. E. Baselga). A, Lesion prior to treatment. B, Echo Doppler image of hemangioma in proliferative phase; note the abundant vascularization. C, Clinical improvement of the nasal hemangioma. D, Decreased vascularization and thickness of the lesion.
In general, the minimum duration of treatment with propranolol should be at least 6 months to avoid regrowth of the tumor mass.23,24
Shi et al.17 assessed the ultrasound characteristics of each phase of infantile hemangioma to decide when to suspend treatment with propranolol. The assessments were carried out at baseline, at 3 months, and at 6 months of treatment. Treatment was suspended at 6 months if internal flow was no longer observed or if flow was normal. If flow was still present, treatment continued until flow signals disappeared or after 11 months of treatment, whichever came sooner.
Chang et al.25 reported a more extensive study of 679 children, in which lesion thickness was also used as the endpoint to decide on discontinuation of treatment. The authors discontinued treatment when the color Doppler evaluation had remained stable for 2 months.
Ultrasound Imaging of Vascular MalformationsVascular malformations are a group of uncommon diseases that affect 0.5% of the population. They arise due to innate errors in embryonic development of blood vessels.
Low-Flow Vascular MalformationsVenous MalformationsVenous malformations are lesions formed from anomalous veins. These lesions have differing degrees of communication with adjacent veins.
Clinically, they present as bluish, soft, depressible tumor masses at a similar temperature to the rest of skin. They increase in size during Valsalva maneuvers. The can occur at any site, with the most frequent being the limbs, head, and neck. Although they are generally asymptomatic, during development, complications such as inflammation and thrombotic episodes may occur.26
Venous malformations are typically solitary lesions, but they can appear in multiple cutaneous and visceral forms. The presence of multifocal venous malformations is suggestive of a hereditary disorder or syndrome. Anomalies of the deep venous system are present in 47% of patients with very extensive venous malformations of the limbs and with Klippel-Trenaunay syndrome. Such findings require study of the deep venous system before initiating treatment.27
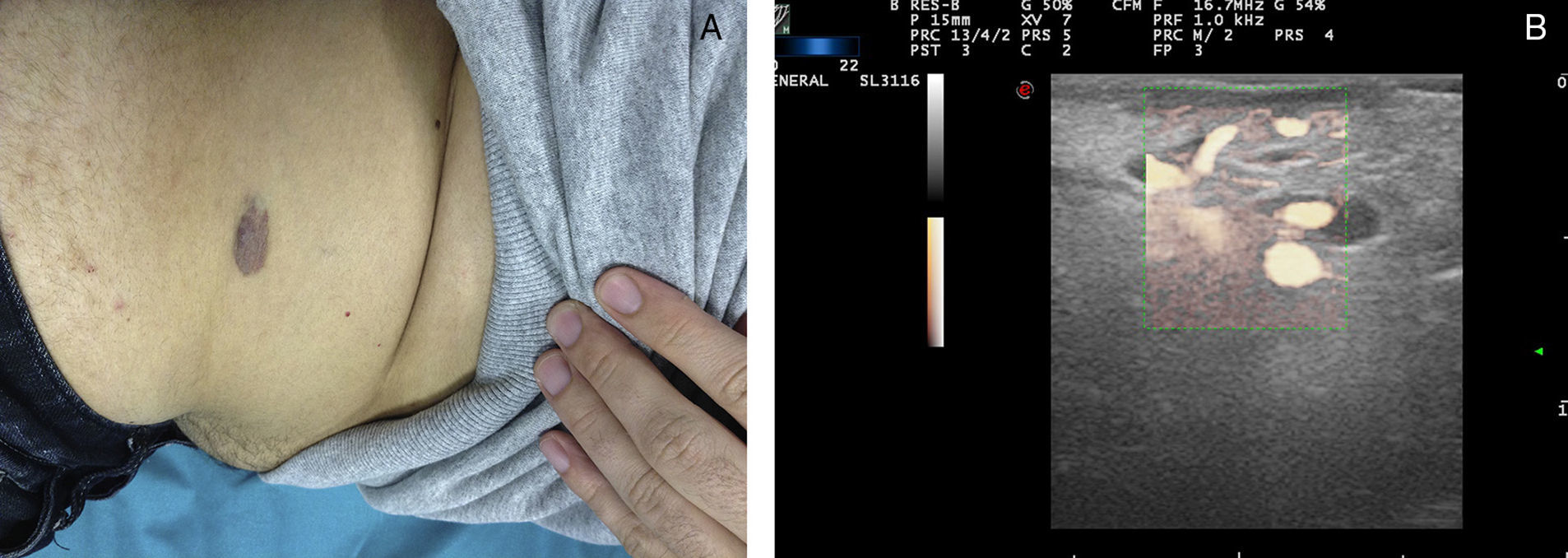
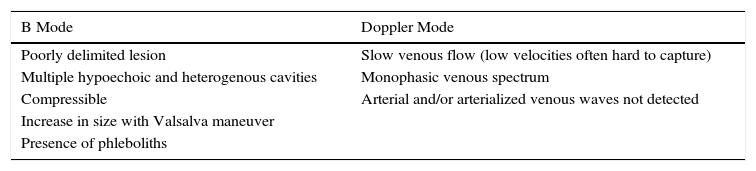
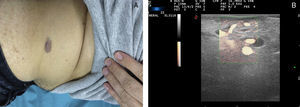
Ultrasound Imaging of Venous MalformationsVenous malformations typically present as anechoic or hypoechoic structures in B mode. Typically, venous malformations have scant fibrous stroma, but the walls of the isolated cavities range from very fine to very thick septa, and so up to 80% of these lesions have a mixed pattern of hypoechogenic cavities and hyperechogenic septa (Fig. 4),28 with the occasional presence of phleboliths.
At times, venous malformations are filled with thrombotic material. In these cases, the ultrasound image appears as a soft tissue tumor with mixed echogenicity, making it difficult to differentiate between hemangiomas and other soft tissue tumors (Table 3).29
Ultrasound Characteristics of Venous Malformations.
| B Mode | Doppler Mode |
|---|---|
| Poorly delimited lesion | Slow venous flow (low velocities often hard to capture) |
| Multiple hypoechoic and heterogenous cavities | Monophasic venous spectrum |
| Compressible | Arterial and/or arterialized venous waves not detected |
| Increase in size with Valsalva maneuver | |
| Presence of phleboliths |
In color Doppler mode, venous malformations have low and slow flows which are more marked with Valsalva maneuvers or compression-decompression. Venous malformations usually have a venous phasic spectrum with no arterial or arterialized venous flows within; these are more characteristic of arteriovenous malformations.
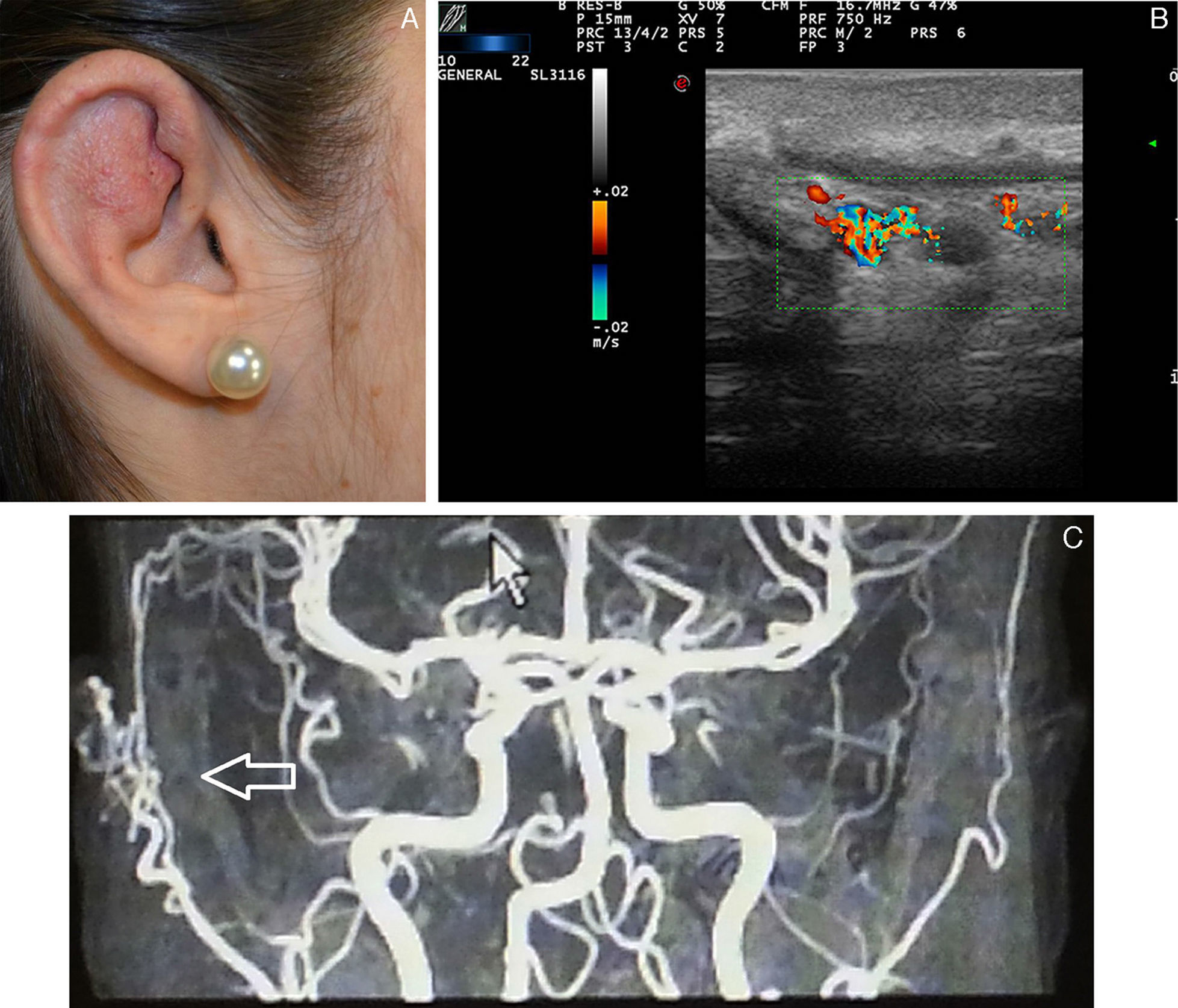
Capillary or Venular Vascular MalformationsCapillary malformations are those in which the predominant vessels are arterioles or postcapillary venules, that is, vessels with a small diameter and slow flow.
The port wine stain is present in 0.4% of newborn children, with no differences between sexes. In 83% of cases, the lesion is present on the head and neck, and interestingly seems to affect the right side of the face to a greater extent than the left. The capillary malformations become raised and darken with age, taking on cobblestone appearance. When the second branch of the trigeminal nerve is involved, the gingival and maxillary mucosa are involved, leading to separation of the teeth and an increased volume of the affected lip.30
Diagnosis is clinical, although when the capillary malformation is located on the face, MRI should be performed to rule out Sturge-Weber syndrome. In a recent study of 289 patients with capillary malformation in the facial region, 15 (5%) were diagnosed with Sturge-Weber syndrome. This risk increases still further if the capillary malformation is located in the first branch of the trigeminal nerve (7%-28%).31
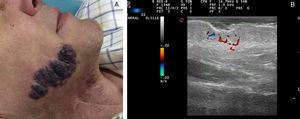
Ultrasound Imaging of Capillary MalformationsIn ultrasound imaging, the capillary malformations appear as hypoechoic dermal areas with limited features in B mode (Fig. 5). In Doppler mode, an increase in Doppler flow may appear within the lesion compared to the adjacent dermis (Table 4).32
Troilius et al.32 presented a study in which 55 patients with port wine stains were assessed by ultrasound imaging. The objective of that study was to assess the depth of the capillary malformation and correlate this with response to treatment with pulsed dye laser, the treatment of choice in these patients.
Given that pulsed dye laser penetrates to a depth of around 0.65mm, the thickness of the capillary malformation may be predictive of response.5
Of the syndromes associated with capillary malformations, dominant autosomal syndromes arising from RAS/MAPK mutations are of particular interest.33 These syndromes have been linked to multifocal cutaneous capillary malformations associated with vascular malformations and arteriovenous fistulas in other territories.34
Kim et al.31 have recently described the presence of arterial flow in the capillary malformations detected by echo Doppler imaging, and they put forward the hypothesis that they could be a manifestation of an underlying atriovenous malformation.
Lymphatic malformations have been reported in individuals with RASA-1 mutations. In this case, cutaneous ultrasound could also be useful for diagnosis.34,35
Lymphatic MalformationsLymphatic malformations are abnormalities in the embryogenesis of lymph vessels. They are classified according to size as microcystic (less than 2cm) and macrocystic (greater than 2cm) and according to their localization as axial or extra-axial.36
Ultrasound Imaging of Lymphatic MalformationsLymphatic malformations have a very characteristic morphology. They are comprised of cystic cavities with shared hyperechoic walls. The material inside the lesion is anechoic, unless an intralesional hemorrhage has occurred. In this case, we can observe hyperechoic content with hyperechoic signals inside the lesion (Table 5).
From the hemodynamic point of view, in general, lymphatic malformations do not have Doppler flow and, if observed, this is usually present at the walls (Fig. 6).
Differential diagnosis should be established with other low-flow vascular anomalies, which can present a similar appearance in the ultrasound study. In these cases, other methods such as MRI are needed for complete anatomical characterization and to determine the involvement of other structures.
The most widely used treatment for this type of malformation is sclerotherapy with agents such as tetracyclines or ethanol. These procedures are usually guided by ultrasound and fluoroscopic imaging.37,38
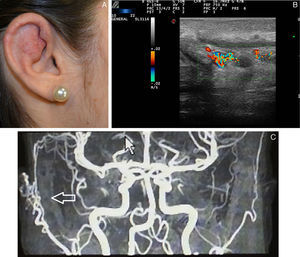
High-Flow Vascular Malformations: Arteriovenous MalformationsArteriovenous malformations are rare in children compared to low-flow lesions. Cutaneous arteriovenous malformations, although always present from birth, are rarely symptomatic at birth or in the early years of life.
Clinically, they manifest as rose-colored macules that resemble an unremarkable capillary malformation. In this state, they are usually asymptomatic and remain this way until adolescence, and some lesions may last a lifetime. Some become more prominent, with more dilated vessels, with vibration and beating (fremitus).
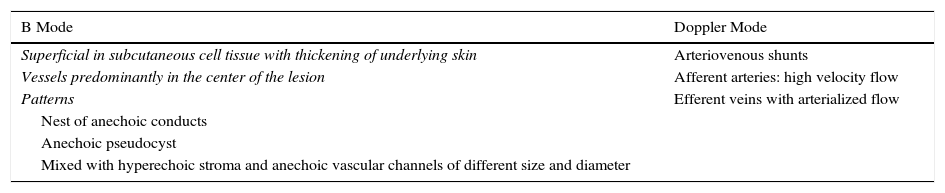
Ultrasound Imaging of Arteriovenous MalformationsUltrasound imaging is the first complementary diagnostic method in these types of lesions.38 In B mode, we observe tortuous, dilated and poorly defined vessels which, unlike those of hemangiomas, do not have the appearance of a tumor mass (Fig. 7).36
The Doppler study reveals vessels with high flow that are not usually observed in venous and lymphatic malformations (Table 6).
Ultrasound Characteristics of Arteriovenous Malformations.
| B Mode | Doppler Mode |
|---|---|
| Superficial in subcutaneous cell tissue with thickening of underlying skin | Arteriovenous shunts |
| Vessels predominantly in the center of the lesion | Afferent arteries: high velocity flow |
| Patterns | Efferent veins with arterialized flow |
| Nest of anechoic conducts | |
| Anechoic pseudocyst | |
| Mixed with hyperechoic stroma and anechoic vascular channels of different size and diameter |
The technique that best enables us to visualize arteriovenous malformations is contrast-enhanced MRI. However, sometimes this technique may not permit venous drainage to be assessed and arteriography is necessary.37
Ultrasound Imaging of Other Vascular AnomaliesUltrasound imaging of other vascular anomalies has been less extensively studied and their characteristics have been extracted from the isolated case reports available.
Kaposiform Hemangioendothelioma and Tufted AngiomaKaposiform hemangioendothelioma and tufted angiomas appear to lie at the ends of the same tumor spectrum, with tufted hemangioma the surface variant and kaposiform hemangioendothelioma the deeper version.39
In view of their proliferative capacity, they can cause arteriovenous fistulas that may lead to vascular entrapment phenomena with hemolytic anemia, platelet destruction, and disseminated intravascular coagulation (Kasabach-Merritt phenomenon).40
In ultrasound imaging, high-flow hyperechoic lesions with a solid appearance have been reported in cases with cutaneous or mucosal expression.41
The presence of arteriovenous fistulas in these lesions is not necessarily associated with the presence of the Kasabach-Merritt phenomenon,42 as demonstrated in the series of 9 cases from the General Hospital in Valencia, Spain. Although arteriovenous fistulas were present in several of their cases, this phenomenon was not observed in any of them.
AngiosarcomaThe ultrasound imaging reports of angiosarcomas refer to mammary lesions,43 which are rarely present in primary cutaneous angiosarcomas. The mammary variant of angiosarcoma has variable ultrasound characteristics and can appear either as hyperechoic lesions with lobulated borders or, less frequently, as lesions with mixed echogenicity with hyperechoic areas and irregular borders.44
ConclusionsAt present, ultrasound is the first diagnostic study necessary in the assessment of vascular anomalies. This study should be requested by the dermatologist who coordinates the multidisciplinary management of these lesions.
Even if the dermatologist does not actually perform the procedure, he or she should know:
- 1)
What type of ultrasound imaging has been used (B mode, color and spectral Doppler)
- 2)
What data consistent with the suspected vascular anomaly can be found in the ultrasound report (for example, presence of channels or phleboliths in venous malformations).
- 3)
The limitations of ultrasound imaging for differentiating between these lesions and other similar ones (for example, in capillary malformations), What imaging techniques are complementary to ultrasound study (CT, MRI, conventional X-ray).
Dermatologists’ knowledge of ultrasound imaging would facilitate communication with the radiographer and enable better diagnosis and treatment for patients with vascular anomalies.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Alfageme Roldán F, Salgüero Fernández I, Zamanta Muñoz Garza F, Roustán Gullón G. Actualización en ecografía de las anomalías vasculares. Actas Dermosifiliogr. 2016;107:284–293.