Apremilast is a phosphodiesterase-4 inhibitor taken orally. Little information about its use in routine clinical practice is available. We aimed to assess treatment safety and persistence rates in patients on apremilast for different forms of plaque psoriasis. This observational retrospective study included 30 patients with psoriasis who were treated with apremilast between January 2016 and December 2017 in our hospital. Twelve patients had palmar-plantar psoriasis, 8 had plaque psoriasis mainly on the scalp, and 10 had plaque psoriasis in other locations. The probable period of treatment persistence in patients in the 50th percentile was 18.5 months according to survival analysis of the series overall. Our experience suggests that apremilast is effective and safe for treating palmar-plantar psoriasis and plaques at other locations but not for treating scalp psoriasis. Adverse effects that compromise treatment occur in nearly two-thirds of the patients.
El apremilast es un inhibidor vía oral de la fosfodiesterasa-4 con pocos datos de práctica clínica habitual. Nuestro objetivo fue evaluar la persistencia y la seguridad del apremilast en la práctica clínica en distintas formas clínicas de psoriasis. Se realizó un estudio observacional retrospectivo de los 30 pacientes con psoriasis que recibieron el fármaco entre enero de 2016 y diciembre de 2017 en nuestro centro, 10 con psoriasis en placas, 8 con placas predominantemente en el cuero cabelludo y 12 palmo-plantares. El tiempo global de probabilidad de supervivencia del 50% fue de 18,5meses. En nuestra experiencia, el apremilast es un fármaco efectivo y seguro para la psoriasis en placas y palmo-plantar, no así para la afectación del cuero cabelludo. Los efectos secundarios ocurren en casi dos tercios de los pacientes comprometiendo la persistencia del tratamiento.
Apremilast is an oral phosphodiesterase type 4 (PDE-4) inhibitor that was approved by the US Food and Drug Administration in 2014 and by the European Medicines Agency in 2015 for the treatment of plaque psoriasis in adults. PDE-4 inhibition leads to increased intracellular cAMP, in turn leading to increased antiinflammatory cytokines and decreased proinflammatory cytokines (TNF-alfa, IL-6, IL-17, and IL-23).1,2
Clinical trials (ESTEEM 1 and ESTEEM 2) have demonstrated efficacy, with psoriasis area severity index (PASI) 50 and PASI 75 achieved by 55.5% and 33.1% of patients with plaque psoriasis, respectively.3,4 Subanalysis of the ESTEEM 1 and ESTEEM 2 trials, focusing on those patients who presented with palmoplantar hyperkeratotic lesions in addition to plaques, showed that 48% of patients with a PGA greater than or equal to 3 achieved physician general assessment (PGA) 0/1 after the first 16 weeks of treatment.5 In another subanalysis of the ESTEEM clinical trials on the effectiveness of treatment in scalp psoriasis, 40% to 50% of patients achieved PGA 0/1 at 16 weeks of treatment, with maintenance of response and even improvement at week 52.6 These results, although derived from a subanalysis, suggest that apremilast is an effective drug for treatment of hard-to-treat areas. However, data from clinical practice to support this suggestion are limited. The objective of the present study was to assess the data obtained for persistence and safety in our clinical practice, after 2years of apremilast use in different clinical forms of psoriasis.
Material and MethodsA retrospective observational study was conducted in the Hospital Universitario y Politécnico La Fe in Valencia, Spain. All patients with psoriasis, except for those with pustular forms, were included if they had received at least 1 dose of apremilast between January 2016 and December 2017. Dosing was as per the prescribing information. Given the clinical practice setting, use of topical treatment was permitted, and apremilast could be initiated without the need for a prior washout period.
The persistence with and safety of treatment, as well as the influence on these outcomes of demographic variables (sex, age, weight, height, body mass index [BMI]) and clinical variables (duration of psoriasis, number of prior treatments, and initial PASI and PGA) were analyzed. A patient was considered as belonging to the subgroup with plaques predominantly on the scalp when more than 20% of body area was affected and less than 3% of the lesions affected the trunk and/or limbs. The primary reason for failure was defined as the absence of PASI 50 improvement in plaque psoriasis, or the absence of a decrease of at least 2 points in PGA in palmoplantar (PP) and scalp presentations. The secondary reason for failure was defined as an adequate initial response lost over at least 2 consecutive visits. For all patients, the presence, seriousness, and duration of adverse effects was evaluated.
Descriptive analysis of the variables was performed with absolute and relative frequencies and means and SDs. Inferential analysis of the qualitative variables was performed using chi-squared based tests. Survival analysis was performed using the Kaplan-Meier method and log-rank test, along with the 50th and 75th percentile for calculation of time of probability of survival, with a study event defined as withdrawal of treatment for any reason. The statistical analyses were performed using the SPSS statistical package, with the limit of significance set to P<.05.
All medical histories of the patients were treated anonymously and in accordance with privacy and data protection laws. The study was classified by the Spanish Agency for Evaluation of Medicinal Products as a postauthorization study and was approved by the ethics committee of the Hospital Universitario y Politécnico La Fe, Valencia.
ResultsThe study included 30 patients, 18 women and 12 men, with a mean age of 44 years (range, 22–71 years) and a mean BMI of 28.3kg/m2 (19–38kg/m2). The median duration of follow-up was 9 months (range, 0.5–18 months). Ten patients presented plaque psoriasis, 8 predominantly on the scalp and 12 had PP forms. In 19 patients (63%), the indication for apremilast was based solely on the clinical criterion of hard-to-treat area. The remaining indications were as follows: lack of control with traditional drugs or their contraindication (4/30; 13%), untreated latent tuberculosis infection (2/30; 7%), active hepatitis B (2/30, 7%), Lynch syndrome (1/30, 3%), history of spontaneous bacterial peritonitis (1/30, 3%), and autoimmune hepatitis (1/30, 3%). In 7 patients (23%), apremilast was the drug used as the first systemic treatment. Of the 23 patients (77%) who had received some type of prior systemic treatment, 19 patients had received traditional agents (63%), 3 had received traditional drugs and biologics (10%), and 1 patient only biological agents (3%).
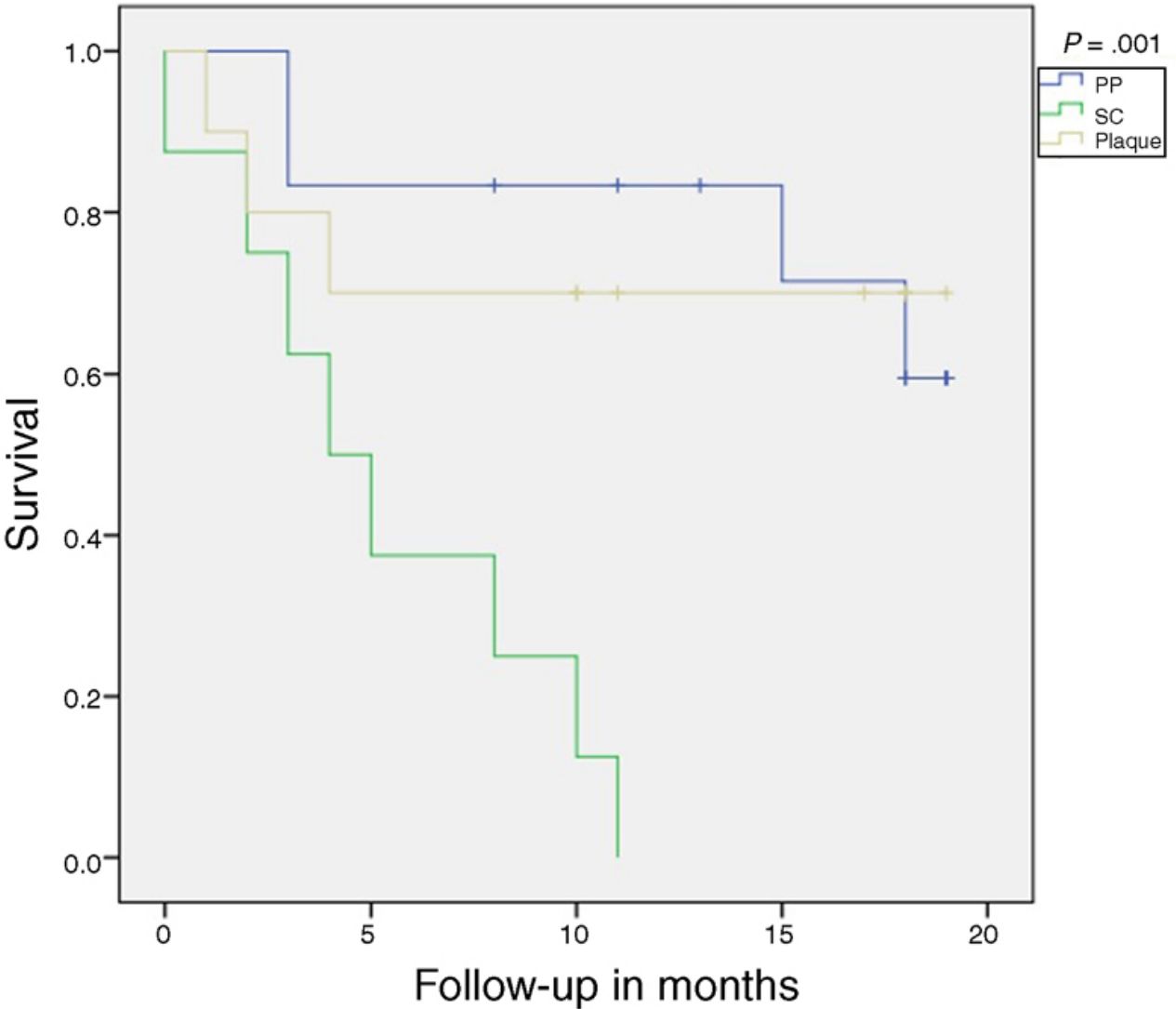
At the end of the study, only 15 patients remained on treatment. The time to 50% survival and 75% survival probability was 18.5 and 4 months, respectively, for all patients, regardless of indication. No patient with plaque psoriasis predominantly on the scalp completed the study period on treatment, with a 50% and 75% survival probability of 5 and 2.5 months, respectively. In plaque psoriasis, the 75% survival probability was 4 months while in the PP form it was 15.6 months. Table 1 shows reasons for withdrawal. Overall, the median time to withdrawal of the drug was 2 months (range, 0.5–4 months) for adverse reactions, 5.5 months (range, 4–8 months) for primary failure, and 14.5 months (range, 11–18 months) for secondary failure. In the 3 types of psoriasis included, we did not identify a statistically significant association between a decrease in PGA≥2 points and BMI, sex, age, and intake of prior treatments. In our study, only the scalp clinical form was significantly associated with withdrawal due to primary and/or secondary failure, as well as lack of decrease of at least 2 points on the PGA (P=.003). Analysis using Kaplan-Meier curves showed a significant reduction in treatment survival in patients with scalp psoriasis (P=.001) (Fig. 1).
Reason for Withdrawal and Proportion of Adverse Drug Reactions.
| All Indications(n=30) | Plaque Psoriasis(n=10) | Palmoplantar Psoriasis(n=12) | Scalp Psoriasis(n=8) | |
|---|---|---|---|---|
| Reason for withdrawal | ||||
| Primary failure | 4 (13%) | 0 | 1 (8%) | 3 (38%) |
| Secondary failure | 4 (14%) | 0 | 2 (16%) | 2 (24%) |
| Adverse effects | 7 (23%) | 3 (30%) | 1 (8%) | 3 (38%) |
| Type of adverse effect | ||||
| Headache | 11 (37%) | 4 (40%) | 5 (42%) | 2 (25%) |
| Diarrhea | 10 (33%) | 4 (40%) | 4 (33%) | 2 (25%) |
| Nausea | 8 (27%) | 1 (10%) | 3 (25%) | 4 (50%) |
| Asthenia | 6 (20%) | 4 (40%) | 2 (17%) | 0 |
| Abdominal pain | 3 (10%) | 0 | 1 (8%) | 2 (25%) |
| Myalgia | 2 (7%) | 2 (20%) | 0 | 0 |
| Cramps | 1 (3%) | 0 | 1 (8%) | 0 |
Twenty-two out of 30 patients (73%) experienced adverse effects related to drug administration; of these, 60% (13/22) had 2 or more adverse effects. In total, 41 adverse effects were reported (Table 1). No patient experienced severe effects or infectious events. In the 15 patients who completed the study, the median duration of side effects was 3.4 months (range, 1–10months), with resolution during the first month of treatment in 6/15 cases (40%), and in the first 2 months in 8/15 (53%). We did not find any statistically significant association between adverse effects and age, BMI, clinical form, response to treatment, or sex. However, of note is a trend (P=.077) between patients with normal weight and the onset of adverse effects, such that 100% of patients with normal weight had adverse effects compared with only 50% of patients with obesity.
DiscussionEfficacy, persistence, and safety of drugs in clinical practice can be different to what is reported in clinical trials.7,8 Therefore, real world studies in a clinical practice setting are necessary. Our results with apremilast in plaque psoriasis were similar to those published recently by Vujic et al.,9 who studied 48 patients in clinical practice for 2 years. The authors did not find any statistically significant differences between drug survival and sex, age, smoking habit, and presence of joint involvement, initial PASI, or prior treatments. They did however find a trend towards an association between the probability of achieving a PASI 75 response or better and BMI less than 30kg/m2. In our series, we only identified a trend for greater risk of adverse effects in patients with normal weight.10 In reference to the adverse effects, our results are similar to those obtained both in clinical trials and other studies in clinical practice settings, with diarrhea, abdominal pain, and headache being the most frequent, occurring in 60% to 80% of patients. It would therefore be advisable to avoid the drug or monitor closely those patients with underlying digestive problems, headaches, or migraines. In our series, we did not report any serious or potentially serious adverse effects, even in patients with underlying infectious disease or neoplasms.
This study is subject to a series of limitations. First, this was a retrospective study, with no control group and a small number of patients, particularly in view of the subsequent subdivision by clinical type. In addition, the concomitant use of topical corticosteroids was not appropriately quantified.
In conclusion, in our experience, apremilast is an effective and safe drug in plaque psoriasis and PP psoriasis. In our series, the clinical forms with predominantly scalp involvement did not respond well to treatment. Adverse effects were reported in nearly two-thirds of the patients, mainly in the form of gastrointestinal conditions or headache, and although most of these were mild or moderate in intensity, they may compromise persistence with treatment.
Conflicts of InterestA. Sahuquillo-Torralba and R. Botella Estrada have worked as consultants and/or received honoraria as speakers and/or participated in clinical trials sponsored by companies that develop drugs used in the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis, and Pfizer.
E. Monte Boquet has worked as a consultant and/or received honoraria as a speaker for companies that develop drugs used in the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis, and Pfizer.
The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Sahuquillo-Torralba A, de Unamuno Bustos B, Rodríguez Serna M, Monte Boquet E, Botella Estrada R. Persistencia y seguridad del apremilast en el tratamiento de la psoriasis en la práctica clínica habitual: experiencia en 30 pacientes. Actas Dermosifiliogr. 2020;111:415–418.