Malignant cutaneous adnexal neoplasms form a group of rare, typically low-grade-malignancy carcinomas with follicular, sebaceous, apocrine, or eccrine differentiation or a combination of the first 3 subtypes. Their clinical presentation is usually unremarkable, and biopsy is required to establish the differentiation subtype and the definitive diagnosis. Due to their rarity, no clear consensus has been reached on which treatment is most effective. Mohs micrographic surgery is considered to be the best option to prevent recurrence in the majority of patients. Radiotherapy and chemotherapy have been studied in very few cases and have rarely been shown to be effective.
Las neoplasias anexiales cutáneas malignas constituyen un grupo de carcinomas poco frecuentes, habitualmente de bajo grado de malignidad, que muestran diferenciación folicular, sebácea, apocrina o ecrina o una combinación de las 3 primeras. Clínicamente suelen ser neoplasias con características poco distintivas, siendo necesaria una biopsia que permitirá establecer el tipo de diferenciación y el diagnóstico definitivo. Al tratarse de una enfermedad poco frecuente, no existe un claro consenso sobre el tratamiento más eficaz. En la mayoría de casos se considera la microcirugía de Mohs como la opción más efectiva para prevenir recidivas. La radioterapia y quimioterapia han sido escasamente estudiadas y solo se han mostrado eficaces en escasas ocasiones.
Malignant cutaneous adnexal neoplasms are an uncommon group of low-grade carcinomas. Although most of these tumors have very limited ability to spread to distant sites, they are locally aggressive and must be treated with surgical excision to ensure tumor-free margins. Malignant cutaneous adnexal neoplasms have distinctive histopathologic characteristics, but their clinical characteristics are largely nonspecific. The type of differentiation present in each tumor is recognizable through histopathologic characteristics that resemble certain findings present in the corresponding normal adnexal structures. Generally speaking, as these tumors are malignant, they show few signs of differentiation. Investigation through serial cuts or immunohistochemical staining is therefore necessary to establish the type of differentiation in a given adnexal tumour. Many malignant cutaneous adnexal carcinomas display only ductal differentiation, and as eccrine and apocrine ducts are currently indistinguishable both immunohistochemically and ultrastructurally, all that can be clearly established in such cases is that the tumour is a ductal carcinoma. No further differentiation is possible. Although differentiation is minimal, however, certain histopathologic features, summarized in Table 1, can suggest malignancy. In this article, we review the malignant cutaneous adnexal neoplasms listed in Table 2.
Histopathologic Differential Diagnosis Between Benign and Malignant Adnexal Neoplasms.
| Benign Tumors | Neoplastic Tumors |
|---|---|
| Symmetric | Asymmetric |
| Well circumscribed | Poorly circumscribed |
| Upturned V-shape often present | Upturned V-shape often absent |
| Frequent vertical orientation | Frequent horizontal orientation |
| Smooth borders | Serrated borders |
| Condensed peripheral fibrous tissue | Noncondensed peripheral fibrous tissue |
| Clefting between the tumor stroma and the adjacent healthy dermis | Clefting between the tumor stroma and the epithelium |
| Enucleation often easy following incision | Enucleation often difficult following incision |
| Stroma predominating over epithelium | Epithelium predominating over stroma |
| Tends to be located in superficial layers | Tends to invade deep layers |
| No epidermal ulceration | Frequent epidermal ulceration |
| Tumor islands separated by abundant stroma | Tumor islands separated by scanty stroma |
| Tumor islands with a relatively uniform shape and size | Tumor islands of varying shapes and sizes |
| Small individual tumor islands | Sheets of confluent tumor islands |
| Well differentiated | Poorly differentiated |
| Conservation of existing adnexal structures | Destruction of existing adnexal structures |
| General absence of massive necrosis | Massive necrosis common |
| Non-neoplastic cells in perineural location | Perineural neoplastic cells common |
| Absence of intravascular neoplastic cells | Occasional presence of intravascular neoplastic cells |
| Absence of cords of epithelial cells among collagen bundles | Cords of epithelial cells among collagen bundles |
| Tumor islands tend to become smaller as they penetrate the dermis | Tumor islands do not tend to become smaller as they penetrate the dermis |
Classification of Malignant Adnexal Neoplasms According to Type of Differentiation.
| Malignant adnexal neoplasms with follicular differentiation |
| Pilomatrix carcinoma |
| Malignant adnexal neoplasms with sebaceous differentiation |
| Sebaceous carcinoma |
| Malignant adnexal neoplasms with eccrine or apocrine differentiation |
| Syringocystadenocarcinoma papilliferum |
| Tubular carcinoma |
| Papillary carcinoma |
| Hidradenocarcinoma papilliferum |
| Apocrine hidradenocarcinoma |
| Malignant mixed tumor |
| Malignant cylindroma |
| Spiradenocarcinoma |
| Syringoid carcinoma |
| Porocarcinoma |
| Microcystic adnexal carcinoma |
| Adenoid cystic carcinoma |
| Mucinous carcinoma |
| Signet-ring cell carcinoma |
| Extramammary Paget disease |
Pilomatrix carcinoma presents as a solitary nodule on the upper body in the vast majority of cases.1 It is more common in middle-aged and elderly men. Most publications to date have described a largely nonaggressive biologic behavior, but there have been reports of metastasis to regional lymph nodes and internal organs with fatal outcomes.2
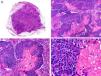
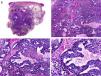
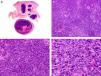
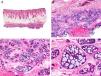
Histopathologically, pilomatrix carcinoma is seen as an asymmetric, poorly circumscribed tumor with frequent ulceration of the epidermal surface. It consists of a proliferation of immature basaloid cells, reminiscent of follicular matrical cells, that form either solid basaloid islands or cords of cells penetrating the deep dermis, subcutaneous tissue, and even the fascia and muscle (Fig. 1). The most common finding is the presence of islands of shadow cells indicating matrical differentiation.3
Histopathologic characteristics of pilomatrix carcinoma. A, Panoramic view showing a lesion formed by numerous tumor islands invading the dermis. B, Islands of matricial cells containing shadow cells in the center. C, Detail of matricial and shadow cells. D, High-magnification view of neoplastic cells with matricial differentiation. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Pilomatrix carcinoma is a malignant tumor that frequently shows aggressive local behavior. It invades adjacent structures, and incomplete excision of the primary tumor results in disease persistence in 60% of cases.4 Metastasis generally occurs through hematogenous or lymphatic spread and there have been reports of distant metastasis resulting in death.5 Most publications recommend surgical excision with margins of between 5mm and 2cm.6,7 Mohs micrographic surgery (MMS) is a good option as it frequently achieves clear margins. Adjuvant radiation therapy has produced varying results, but has shown no clear improvement in recurrence rates. Intravenous chemotherapy has not proven effective.8,9 Due to the high rates of recurrence and metastasis, mostly to lymph nodes, patients should be scheduled for clinical check-ups including regional lymph node examination every 4 to 6 months.9
Sebaceous CarcinomaSebaceous carcinoma is classified as ocular or extraocular. The distinction is based not only on anatomic location but also on the greater metastatic potential of the ocular variant, although this has recently been questioned.10
Sebaceous carcinoma of the eyelid generally affects elderly patients and is frequently interpreted as an inflammatory lesion, resulting in a delayed diagnosis. Most cases of extraocular sebaceous carcinoma occur on the face and neck of elderly patients. Clinically, the lesion presents as a nodule or indurated plaque that may be ulcerated.
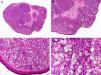
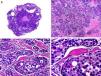
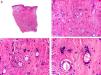
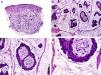
Histopathologically, sebaceous carcinoma presents as a poorly circumscribed tumor formed by islands of epithelial cells that invade the dermis or the chorion of the conjunctival mucosa in the case of eyelid involvement. Subcutaneous tissue involvement is also common (Fig. 2). Cytologic degree of sebaceous differentiation is variable. Some tumors show immature basaloid cells, while others show neoplastic cells with morphologic features of mature sebocytes, a cytoplasm containing numerous lipid vacuoles, and a retracted nucleus with spiculated borders due to the pressure exerted by the lipid vacuoles on the nuclear membrane. Detection of ducts with a similar morphology to that of sebaceous ducts, with a serrated cuticle, can aid diagnosis.11 Adipophilin is a very useful immunohistochemical marker for identifying sebaceous differentiation in formalin-fixed or even paraffin-based tumor specimens.12
Histopathologic characteristics of sebaceous carcinoma. A, Panoramic view showing islands of neoplastic cells invading the full thickness of the dermis. B, Several neoplastic aggregates showing a tendency to converge. C, Detail of sebaceous differentiation in the form of sebocytes at different stages of maturation. D, Sebocytes at different stages of maturation. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Surgical excision is the treatment of choice for sebaceous carcinoma, although some authors have suggested that MMS is a superior option.13 Adjuvant radiation therapy in cases of recurrence or metastasis has proven effective.14 Muir-Torre syndrome must be ruled out in all patients diagnosed with sebaceous carcinoma.15
Syringocystadenocarcinoma PapilliferumMost cases of syringocystadenocarcinoma papilliferum appear to arise from the malignant transformation of an existing syringocystadenoma papilliferum and almost always against the background of nevus sebaceous of Jadassohn.16 Syringocystadenocarcinoma papilliferum is more common in women and has a predilection for the scalp. Clinically, it presents as a plaque consisting of multiple confluent yellowish papules, some of which may be ulcerated or crusted.
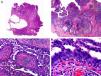
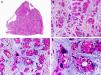
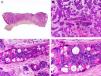
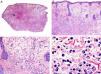
Histopathologically, syringocystadenocarcinoma papilliferum is similar to its benign counterpart, as it is formed by large papillary structures. Unlike syringocystadenoma papilliferum, however, it appears as an asymmetric, poorly circumscribed tumor that invades the hypodermis and underlying tissues. Papillary structures penetrate cystic cavities, which are connected to the skin surface through pre-existing infundibular structures and are lined by an epithelium with clear signs of atypia and frequent mitotic figures (Fig. 3).17
Histopathologic characteristics of syringocystadenocarcinoma papilliferum. A, Panoramic view showing neoplastic aggregates of varying shapes and sizes invading the dermis. B, Papillary structures connected to the epidermal surface. C, Note how these papillary structures are lined with a double layer of epithelial cells. D, Images of nuclear atypia and pleomorphism in the epithelial cells lining the papillae. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Syringocystadenocarcinoma papilliferum is a low-grade adenocarcinoma. Regional lymph node metastasis has been described in 5 of the 36 cases reported in the literature, and just 1 of these led to death.18–22 MMS has shown good results, but surgical excision with wide margins continues to be the treatment of choice. The effectiveness of radiation therapy is not clear as varying results have been reported.22,23
Tubular CarcinomaApocrine tubular carcinoma is more common in middle-aged women. It appears as a firm subcutaneous nodule sometimes fixed to the underlying tissue. It has a predilection for the axillae.
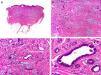
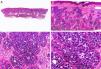
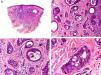
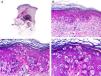
Histopathology shows multiple, closely packed ductal structures occupying the full thickness of the dermis and the subcutaneous tissue. As the lesion penetrates into the dermis, the glandular lumina and neoplastic aggregates become progressively smaller (Fig. 4). Before making a diagnosis of primary tubular carcinoma, it is necessary to rule out a cutaneous metastasis from a visceral adenocarcinoma or a tubular carcinoma in the axillary tail of the breast.
Histopathologic characteristics of tubular carcinoma. A, Panoramic view showing tubular structures invading the dermis. B, Tubular structures immersed in a sclerotic stroma. C, Tubular structures of varying shapes and sizes. D, Signs of decapitation secretion in one of the tubules. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Tubular carcinoma must be treated by complete surgical excision. Like other adnexal carcinomas, it shows malignant behavior and there have been several reports of distant metastasis and death due to disease spread.24
Papillary Carcinoma (Aggressive Digital Papillary Adenocarcinoma)Papillary carcinoma is more common in men and has a clear predilection for the fingers, where it presents as a firm, sometimes ulcerated, subcutaneous nodule.
Despite its name, papillary carcinoma is a predominantly solid tumor. The only predominant focal features are tubular structures containing papillae.25 Histopathologically, neoplastic cells appear as basaloid cells with a hyperchromatic nucleus that tend to form solid aggregates (Fig. 5).
Histopathologic characteristics of papillary carcinoma. A, Panoramic view showing a tumor invading the full thickness of the dermis and extending into the subcutaneous tissue. B, Neoplastic aggregates of varying shapes and sizes. C, Traces of papillary structures in some of the neoplastic aggregates. D, Detail of the papillary structures. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Papillary carcinoma is a slow-growing tumor with high rates of persistence following incomplete excision. There have been just 3 reports of death due to metastatic spread. The metastasis occurred many years after diagnosis, demonstrating the low-grade nature of this tumor.26 Recurrence can occur in up to 50% of patients who undergo conservative excision, but this rate can be as low as 5% in cases of radical excision or finger amputation.27 As metastasis is common, patients should undergo a regional lymph node study and chest radiography following diagnosis and also be referred for annual check-ups over a period of 10 years. The value of sentinel lymph node biopsy has not been confirmed in this setting.
Hidradenocarcinoma PapilliferumHidradenocarcinoma papilliferum frequently arises in an existing hidradenoma papilliferum. Most cases to date have been described in the anogenital region of middle-aged women. The lesions present as protruding tumoral lesions or subcutaneous nodules, sometimes accompanied by an ulcer with a bloody exudate. In most cases, they are clinically interpreted as superinfected cysts.
When viewed under low-power magnification, hidradenocarcinoma papilliferum has a similar appearance to that of hidradenoma papilliferum, as it is formed by a cystic structure with papillary structures protruding into the cyst. Careful examination, however, shows an irregular architecture with invasion of adjacent tissue. The papillary structures are composed of a central core of fibrovascular tissue lined by a double layer of epithelial cells marked by nuclear pleomorphism and frequent mitotic figures.28
TreatmentHidradenocarcinoma papilliferum has metastatic potential and must be completely excised. Three of the 9 patients described in the literature had regional lymph node metastasis at the time of diagnosis and 2 died as a result of disease spread.
Apocrine HidradenocarcinomaApocrine hidradenocarcinoma is more common in men in their 50s. The tumor presents as an asymptomatic subcutaneous nodule with nonspecific clinical features.
Histopathologically, apocrine hidradenocarcinoma appears as a multilobulated tumor with solid islands of neoplastic cells of varying shapes and sizes that display deep, asymmetric invasion (Fig. 6). Tubular structures are evident in most tumors, and decapitation secretion at the luminal border is occasionally seen.29
Histopathologic characteristics of papillary apocrine hidradenocarcinoma. A, Panoramic view of a tumor invading the full thickness of the dermis. B, Neoplastic aggregates of varying shapes and sizes. C, Note the cells with a pale cytoplasm in some of the neoplastic aggregates. D, Detail of a neoplastic aggregate with ductal differentiation. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Surgical excision is currently the treatment of choice for localized disease. According to the few reports described to date, apocrine hidradenocarcinoma shows aggressive biologic behavior and is characterized by high rates of local recurrence and metastasis with a generally dismal prognosis. There has also been a report of favorable response to chemotherapy and radiation therapy in a patient with metastasis to distant sites.30
Malignant Mixed TumorMalignant mixed tumor has no distinctive clinical characteristics and presents as a subcutaneous nodule with occasional epidermal ulceration. It sometimes invades the underlying tissues and appears fixed to the deep layers. Histopathologically, it displays an irregular architecture with a double epithelial and mesenchymal component. The epithelial component contains ducts and small tubules, while the mesenchymal component is typically myxoid (Fig. 7), although it may be chondroid or osteoid.31
Histopathologic characteristics of malignant mixed tumor. A, Panoramic view showing a poorly circumscribed tumor. B, Aggregates of neoplastic epithelial cells immersed in a myxoid stroma. C, Neoplastic aggregates formed by cells with an atypical, pleomorphic nucleus. D, Signs of ductal differentiation in some of the aggregates. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Over 50% of malignant mixed tumor cases described to date have had associated regional lymph node and distant metastases, which in some cases have led to death. Complete surgical excision prior to metastasis is thus the only curative treatment currently possible.
Malignant CylindromaMalignant transformation of an existing cylindroma is more common than de novo malignant cylindroma and occurs more frequently in patients with multiple lesions. The lesions suddenly exhibit rapid growth and on occasions epidermal ulceration following years of stable disease. In many cases, the tumor will already have invaded the underlying cranial bone by the time the patient is seen.
Histopathologically, malignant cylindroma is seen as multiple islands of basaloid cells arranged in a jigsaw-like pattern (Fig. 8), similar to that seen in its benign counterpart. The benign and malignant variants can coexist in the same lesions and there may even be a gradual transition between the two. Architectural asymmetry and cellular atypia, combined with necrotic areas, are strongly diagnostic of malignant cylindroma.32
Histopathologic characteristics of malignant cylindroma. A, Panoramic view showing a poorly circumscribed tumor invading the full thickness of the dermis. B, The tumor is formed by aggregates of neoplastic basaloid cells. C, Aggregates distributed in a jigsaw-like pattern. D. Detail of one of the neoplastic aggregates surrounded by a thick basement membrane. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Malignant cylindroma is a high-grade tumor that must be treated by surgical excision with wide margins. Distant metastasis has been described in 11 of the 29 cases in the literature, and distant metastasis was responsible for death in 9 of these. MMS has been proposed as the best treatment modality.33
SpiradenocarcinomaSpiradenocarcinoma typically arises in a long-standing spiradenoma that suddenly starts to grow. It is more common on the extremities, although it can affect any part of the body.
Histopathologically, spiradenocarcinoma is classified as poorly or well differentiated. Well-differentiated tumors have similar findings to spiradenoma, with epithelial aggregates of basaloid cells containing multiple ductal structures (Fig. 9). The lesions, however, are asymmetric and poorly circumscribed, and show islands of epithelial cells of greatly varying shapes and sizes, in addition to frequent necrotic areas and invasion of adjacent tissue. In the case of poorly differentiated spiradenocarcinoma, a definitive diagnosis can only be established if traces of spiradenoma are observed in the vicinity of an undifferentiated carcinoma. On occasions, this poorly differentiated tumor may contain spindle cells, leading to confusion with sarcoma.34
Histopathologic characteristics of spiradenocarcinoma. A, Panoramic view showing a spiradenoma in the dermis and a spiradenocarcinoma nodule in the subcutaneous tissue. B, The nodule is formed by a sheet of neoplastic cells. C, Note the pleomorphic nuclei and frequent mitotic figures in the neoplastic cells. D. High-magnification view of neoplastic cells, several of which are in mitosis. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Spiradenocarcinoma is a high-grade tumor and must be completed excised. Distant metastasis has been reported in 12 of the 31 cases in the literature and was directly related to death in at least 5 of these. To establish a prognosis, it is essential to identify the level of differentiation at diagnosis as tumors with a higher degree of differentiation pursue a more indolent course and very rarely metastasize.35
Syringoid CarcinomaSyringoid carcinoma is a slow-growing tumor with a predilection for the head. It presents as a subcutaneous nodule or plaque that is noticeably hard on palpation.
Histopathologically, it is characterized by multiple ductal structures and small cysts that occupy the full thickness of the dermis and frequently invade the subcutaneous tissue, destroying adjacent structures36 (Fig. 10).
Histopathologic characteristics of syringoid carcinoma. A, Panoramic view showing a poorly circumscribed tumor invading the full thickness of the dermis. B, The neoplasm is formed by small ductal structures immersed in a sclerotic stroma. C, These ductal structures are lined with a layer of epithelial cells, some of which have an epithelial tadpole shape. D, High-magnification view of epithelial cells lining the ducts with no signs of cellular atypia. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Syringoid carcinoma is a locally destructive tumor that must be treated with complete surgical excision. MMS is the modality of choice and is associated with more favorable outcomes, except in cases where the tumor appears to spare certain areas of the dermis, which contain no signs of neoplastic aggregates. It is important not to confuse these cases with multifocal tumors. Chemotherapy and radiation therapy have largely been used to treat metastatic syringoid carcinoma, and there have also been reports of local control with radiation therapy.36–39 There is little experience with chemotherapy, and temporary remission has been reported in just a few cases.37–41
PorocarcinomaPorocarcinoma affects adults and elderly patients. Its most common presentation is a warty or sometimes ulcerated nodular or tumor lesion on the lower extremities (Fig. 9). Most porocarcinomas are de novo tumors, although there have been reports of malignant transformation of a long-standing poroma.
Histopathologically, porocarcinoma has typical architectural features of malignancy, with an asymmetric, poorly circumscribed tumor of varying shapes and sizes. The tumor is composed of 2 types of neoplastic cells: poroid and cuticular. Some of the neoplastic aggregates feature small ductal structures surrounded by cuticular cells (Fig. 11). On occasions, the tumor may be so poorly differentiated that it is impossible to distinguish between the 2 cell types. Epidermotropism is common and in some cases is so marked that it is very difficult to determine histologically whether the porocarcinoma is a primary lesion or an epidermotropic metastasis.42
Histopathologic characteristics of porocarcinoma. A, Panoramic view showing an ulcerated tumor invading the full depth of the dermis. B, Neoplastic aggregates of varying shapes and sizes. C, Signs of ductal differentiation in some of the neoplastic aggregates. D, Detail of small ducts lined by cuticular cells. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Approximately 20% of porocarcinomas are associated with regional lymph node metastasis,42 which in turn is associated with a mortality of 67%.42 Excision by MMS is the treatment of choice and has been found to result in lower rates of recurrence and metastasis.43 A histogenetic role has been attributed to HRAS and EGFR in some variants of porocarcinoma, indicating a possible role for targeted therapies in the near future.44
Microcystic Adnexal CarcinomaMicrocystic adnexal carcinoma is a slow-growing tumor that preferentially affects the skin in the nasolabial and periorbital areas.45 It presents as a firm solitary nodule or plaque with a normal, atrophic, scaling, or on rare occasions, ulcerated surface.
Histopathologically, microcystic adnexal carcinoma penetrates into the deep dermis (Fig. 12) and frequently invades the subcutaneous tissue and sometimes even the underlying fascia and skeletal muscle. Perineural invasion is common in deep components of the lesion. Microcystic adnexal carcinoma has 3 distinct components arranged in horizontal layers. The surface areas contain cystic structures surrounded by squamous eosinophilic and/or pale cells, while the central layers contain solid islands of pale or eosinophilic cells of varying shapes and sizes interspersed with small, round ductal structures immersed in a sclerotic stroma. The deep layers, in turn, contain long tubular structures filled with homogeneous eosinophilic material. All these epithelial structures are immersed in a densely desmoplastic or sclerotic stroma.45
Histopathologic characteristics of microcystic adnexal carcinoma. A, Panoramic view showing a tumor invading the full thickness of the dermis. B, Solid aggregates and small keratin-containing cysts. C, Note the tiny ductal structures in some of the neoplastic aggregates. D, Detail of small ductal structures in some of the neoplastic aggregates. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Microcystic adnexal carcinoma is a locally aggressive tumor. MMS is the treatment of choice, as the tumor has poorly circumscribed borders and invades the subcutaneous tissue, the skeletal muscle, and even bone.46 Because of its stroma, microcystic adnexal carcinoma is relatively resistant to radiation therapy and there has been no experience with chemotherapy to date.47 Adjuvant radiation therapy is an option for areas in which clear tumors margins have not been achieved.48 While metastasis is very rare, recurrence is common and has been reported as long as 30 years after the excision of the original tumor, highlighting the importance of long-term follow-up.46
Adenoid Cystic CarcinomaAdenoid cystic carcinoma appears as a solitary dermal nodule or as multiple nodules that converge to form an indurated plaque that frequently invades the subcutaneous tissue and is fixed to the deep layers. Its most common location is the scalp, but it can occur in other parts of the body.
Histopathologically, primary adenoid cystic carcinoma appears as a poorly circumscribed tumor with aggregates of epithelial cells invading the deep layers. These aggregates, which are composed of basaloid cells, vary greatly in size and shape from one area to the next (Fig. 13) and almost always display perineural and/or endoneural invasion in the deep components of the tumor. The neoplastic cells form an alternating solid and cribriform pattern. Basement membrane deposits are frequently seen throughout the thickness of the tumor.49
Histopathologic characteristics of adenoid cystic carcinoma. A, Panoramic view showing a poorly circumscribed tumor invading the subcutaneous facia, B, Higher-magnification view showing an adenoid cystic pattern in the neoplastic cells. C, Neoplastic aggregates of varying shapes and sizes with an adenoid cystic pattern. D, Higher-magnification view of neoplastic cells. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Cutaneous adenoid cystic carcinoma is a low-grade carcinoma that typically causes local destruction, although it also has metastatic potential. The tumor should be surgically excised with margins of at least 2cm49 or, preferably, treated with MMS.50 Adjuvant and even primary radiation therapy have proven to be of value in tumors that are not candidates for surgical excision or that show perineural invasion.51
Mucinous CarcinomaPrimary cutaneous mucinous carcinoma is somewhat more common in men and is almost always located on the head. It generally presents as a solitary nodule. The epidermal surface varies in appearance but ulcers are rare. Transillumination can be of considerable diagnostic aid as lesions with tumor stroma containing large amounts of mucin are seen as transparent.
Histopathologically, primary cutaneous mucinous carcinoma has a highly characteristic architecture formed by small islands of neoplastic basaloid cells surrounded by pools of mucin separated by thin connective septae causing compartmentalization of the tumor (Fig. 14). Occasional findings include neoplastic epithelial aggregates in the deep dermis or subcutaneous tissue located at some distance from the main tumor component. This separation explains why mucinous carcinoma tends to persist following incomplete surgical excision.
Histopathologic characteristics of mucinous carcinoma. A, Panoramic view showing a tumor with abundant myxoid stroma. B, Thin walls of connective tissue compartmentalizing the tumor. C, The neoplastic cells are basaloid cells and are surrounded by myxoid stroma. D, Higher-magnification view of the neoplastic cells. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Primary cutaneous mucinous carcinoma is rare, and most cases involving the skin are metastases from other sites. There are currently no histopathologic or immunohistochemical techniques for distinguishing a primary cutaneous mucinous carcinoma from a metastasis. This distinction, however, is very important as cutaneous metastases from mucinous carcinoma indicate a very poor prognosis, while primary cutaneous tumors generally have an indolent biologic behavior, although they can metastasize to the regional lymph nodes. All patients with cutaneous mucinous carcinoma must undergo full evaluation to rule out metastasis from a visceral mucinous carcinoma, in particular those involving the breast or colon. Recent studies have suggested that primary cutaneous mucinous carcinoma and cutaneous metastases may have a different cytokeratin pattern.52
TreatmentPrimary cutaneous mucinous carcinoma is a low-grade tumor that is locally destructive but rarely metastasizes. Surgical excision with margins of at least 1cm is associated with a recurrence rate of approximately 34%, although this may drop to 13% when MMS is used.53
Signet-Ring Cell CarcinomaSignet-ring cell carcinoma is a rare tumor that largely affects elderly patients. It occurs mainly on the eyelids but it can also affect the axillae. It presents as a nodule or as diffuse thickening of the skin, a sign that is often interpreted as an inflammatory process.
Signet-ring neoplastic cells are the most distinctive finding in this tumor (Fig. 15). The cells are relatively monomorphic and show no evident atypia. As a result, they may be misinterpreted as squamous histiocytes mimicking an inflammatory process.54 The tumor is formed by cords of cells, small solid islands, and even some neoplastic cells immersed in a sclerotic stroma.
Histopathologic characteristics of signet-ring cell carcinoma of the eyelid. A, Panoramic view showing a poorly circumscribed tumor invading the full thickness of the dermis. B, The tumor is formed by isolated cells scattered through the dermis. C, Note the myxoid stroma in some areas of the tumor. D, High-magnification view of neoplastic cells with a signet-ring morphology. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Before establishing a diagnosis of primary signet-ring cell carcinoma, it is necessary to rule out eyelid metastasis from a primary tumor in another location.
TreatmentSignet-ring cell carcinoma has high potential to produce distant metastasis.39 Choice of treatment is determined by the location of the tumor. Wide excision is relatively simple in axillary tumors, but in tumors involving the eyelid it often requires orbital exenteration. MMS has proven to be more effective at achieving tumor-free margins. Adjuvant radiation therapy appears to be useful for cases in which clear margins are not possible.55
Extramammary Paget DiseaseExtramammary Paget disease is more common in elderly women. It presents as an erythematous macule or plaque with clear borders and a scaling surface, and is preferentially located in the genital area.
Histopathologically, extramammary Paget disease is characterized by intraepithelial Paget cells, which are large cells with a wide, pale cytoplasm and a round, pleomorphic nucleus containing prominent nucleoli. The cells may show occasional signs of cytoplasmic vacuolization and even intraepidermal glandular formations. They have a characteristic distribution marked by small groups of isolated cells scattered through the layers of the epidermis and the adnexa, forming a pagetoid pattern (Fig. 16).
Histopathologic characteristics of extramammary Paget disease. A, Panoramic view showing an intraepidermal lesion. B, The tumor is formed by isolated neoplastic cells scattered through the dermis. C, Note the pleomorphic nucleus and abundant pale cytoplasm in the neoplastic cells. D, Detail of neoplastic cells scattered through the epidermis. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).
Extramammary Paget disease has an indolent biologic behavior characterized by frequent recurrence. Very few cases of lymph node metastasis have been reported, and they have only occurred in long-standing lesions.56 Complete excision is the treatment of choice but this is not always effective, as the lesion often contains tumor-free areas dotted through the epidermal proliferation.57,58 MMS is the treatment of choice,59 although favorable outcomes have also been reported for topical imiquimod 5% and tazarotene,60 local radiation therapy,61,62 and photodynamic therapy.63 Epidermal acantholysis,58 dermal or lymphovascular involvement,64 and Her2/neu positivity65 have been proposed as risk factors for recurrence.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bernárdez C, Requena L. Tratamiento de las neoplasias anexiales cutáneas malignas. Actas Dermosifiliogr. 2018;109:6–23.





![Histopathologic characteristics of pilomatrix carcinoma. A, Panoramic view showing a lesion formed by numerous tumor islands invading the dermis. B, Islands of matricial cells containing shadow cells in the center. C, Detail of matricial and shadow cells. D, High-magnification view of neoplastic cells with matricial differentiation. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of sebaceous carcinoma. A, Panoramic view showing islands of neoplastic cells invading the full thickness of the dermis. B, Several neoplastic aggregates showing a tendency to converge. C, Detail of sebaceous differentiation in the form of sebocytes at different stages of maturation. D, Sebocytes at different stages of maturation. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of syringocystadenocarcinoma papilliferum. A, Panoramic view showing neoplastic aggregates of varying shapes and sizes invading the dermis. B, Papillary structures connected to the epidermal surface. C, Note how these papillary structures are lined with a double layer of epithelial cells. D, Images of nuclear atypia and pleomorphism in the epithelial cells lining the papillae. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of tubular carcinoma. A, Panoramic view showing tubular structures invading the dermis. B, Tubular structures immersed in a sclerotic stroma. C, Tubular structures of varying shapes and sizes. D, Signs of decapitation secretion in one of the tubules. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of papillary carcinoma. A, Panoramic view showing a tumor invading the full thickness of the dermis and extending into the subcutaneous tissue. B, Neoplastic aggregates of varying shapes and sizes. C, Traces of papillary structures in some of the neoplastic aggregates. D, Detail of the papillary structures. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of papillary apocrine hidradenocarcinoma. A, Panoramic view of a tumor invading the full thickness of the dermis. B, Neoplastic aggregates of varying shapes and sizes. C, Note the cells with a pale cytoplasm in some of the neoplastic aggregates. D, Detail of a neoplastic aggregate with ductal differentiation. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of malignant mixed tumor. A, Panoramic view showing a poorly circumscribed tumor. B, Aggregates of neoplastic epithelial cells immersed in a myxoid stroma. C, Neoplastic aggregates formed by cells with an atypical, pleomorphic nucleus. D, Signs of ductal differentiation in some of the aggregates. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of malignant cylindroma. A, Panoramic view showing a poorly circumscribed tumor invading the full thickness of the dermis. B, The tumor is formed by aggregates of neoplastic basaloid cells. C, Aggregates distributed in a jigsaw-like pattern. D. Detail of one of the neoplastic aggregates surrounded by a thick basement membrane. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr8.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of spiradenocarcinoma. A, Panoramic view showing a spiradenoma in the dermis and a spiradenocarcinoma nodule in the subcutaneous tissue. B, The nodule is formed by a sheet of neoplastic cells. C, Note the pleomorphic nuclei and frequent mitotic figures in the neoplastic cells. D. High-magnification view of neoplastic cells, several of which are in mitosis. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr9.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of syringoid carcinoma. A, Panoramic view showing a poorly circumscribed tumor invading the full thickness of the dermis. B, The neoplasm is formed by small ductal structures immersed in a sclerotic stroma. C, These ductal structures are lined with a layer of epithelial cells, some of which have an epithelial tadpole shape. D, High-magnification view of epithelial cells lining the ducts with no signs of cellular atypia. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr10.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of porocarcinoma. A, Panoramic view showing an ulcerated tumor invading the full depth of the dermis. B, Neoplastic aggregates of varying shapes and sizes. C, Signs of ductal differentiation in some of the neoplastic aggregates. D, Detail of small ducts lined by cuticular cells. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr11.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of microcystic adnexal carcinoma. A, Panoramic view showing a tumor invading the full thickness of the dermis. B, Solid aggregates and small keratin-containing cysts. C, Note the tiny ductal structures in some of the neoplastic aggregates. D, Detail of small ductal structures in some of the neoplastic aggregates. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr12.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of adenoid cystic carcinoma. A, Panoramic view showing a poorly circumscribed tumor invading the subcutaneous facia, B, Higher-magnification view showing an adenoid cystic pattern in the neoplastic cells. C, Neoplastic aggregates of varying shapes and sizes with an adenoid cystic pattern. D, Higher-magnification view of neoplastic cells. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr13.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of mucinous carcinoma. A, Panoramic view showing a tumor with abundant myxoid stroma. B, Thin walls of connective tissue compartmentalizing the tumor. C, The neoplastic cells are basaloid cells and are surrounded by myxoid stroma. D, Higher-magnification view of the neoplastic cells. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr14.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of signet-ring cell carcinoma of the eyelid. A, Panoramic view showing a poorly circumscribed tumor invading the full thickness of the dermis. B, The tumor is formed by isolated cells scattered through the dermis. C, Note the myxoid stroma in some areas of the tumor. D, High-magnification view of neoplastic cells with a signet-ring morphology. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr15.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Histopathologic characteristics of extramammary Paget disease. A, Panoramic view showing an intraepidermal lesion. B, The tumor is formed by isolated neoplastic cells scattered through the dermis. C, Note the pleomorphic nucleus and abundant pale cytoplasm in the neoplastic cells. D, Detail of neoplastic cells scattered through the epidermis. (Hematoxylin-eosin, original magnification ×10 [A], ×40 [B], ×200 [C], ×400 [D]).](https://static.elsevier.es/multimedia/15782190/0000010900000001/v1_201801020547/S1578219017303591/v1_201801020547/en/main.assets/thumbnail/gr16.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



