As one of the most common malignancies, basal cell carcinoma (BCC) has evolved as a global burden with incidence annually rising, especially in the older population. Even though the condition is mostly localized, the nature of the disease is destructive and can evolve as either locally advanced BCC (laBCC) or even more rarely as metastatic BCC (mBCC). There are well-established conventional treatment options for these cases, including surgeries and radiotherapy. However, not all cases are eligible for conventional treatments. Recently, biologic treatment has gained a lot of attention and research. This has led to the development of targeted treatment involving the hedgehog pathway inhibitor (HPI), a key pathogenesis in laBCC and mBCC. There are currently two approved HPIs, vismodegib and sonidegib to treat inoperable laBCC and mBCC. This review seeks to explore the pathophysiology of hedgehog pathway behind the development of BCC, and the current update of the efficacy as well as pharmacokinetics properties of HPIs that led to the ideal treatment for laBCC or mBCC, either as monotherapy or in combination with other conventional therapies.
El carcinoma de células basales (CBC) es una de las neoplasias malignas más frecuentes, por lo que se ha convertido en una importante carga asistencial. Su incidencia se incrementa anualmente, especialmente en la población con mayor edad. A pesar de que generalmente está bien localizado, el CBC tiene la capacidad de destruir tejidos y evolucionar a un CBC localmente avanzado (CBCla) o incluso, aunque de forma más rara, a un CBC metastásico (CBCm). Las opciones terapéuticas convencionales en estos casos están bien establecidas, entre las cuales se incluyen la cirugía y la radioterapia. Sin embargo, no todos los casos son elegibles para realizar un tratamiento de tipo convencional. Recientemente, los tratamientos biológicos vienen ganando una mayor atención y son objeto de diversos estudios de investigación. De este modo se ha desarrollado una terapia dirigida utilizando los inhibidores de la vía de Hedgehog (IVH), teniendo en cuenta que se trata de una vía patogénica clave tanto en el CBCla como en el CBCm. En la actualidad, para poder tratar el CBCla y el CBCm no operables existen 2 IVH aprobados: el vismodegib y el sonidegib. Esta revisión busca explorar la fisiopatología de la vía del Hedgehog responsable del desarrollo del CBC y hacer una actualización en cuanto a la eficacia, así como de las propiedades farmacocinéticas de los IVH, características que los convirtieron en la opción terapéutica ideal en el CBCla o en el CBCm, ya sea en forma de monoterapia o en combinación con alguno de los tratamientos convencionales.
Basal cell carcinoma (BCC) is the most common cutaneous cancer in human history. It affects over 2,000,000 people in the United States of America (USA) alone annually.1,2 BCC accounts for more than 80% of all non-melanoma skin cancer (NMSC) and is more prominent in Caucasian people with Fitzpatrick Type I and II skin phototypes and the number of cases worldwide are increasing every year.3,4 Australia is currently leading the highest number of BCC in the world, with incidence of 1531 per 100,000 people.5
Typically, BCC is a relatively indolent localized condition characterized with small to medium, well-defined tumors. Well-established treatment options include surgical and non-surgical approaches. The gold standard treatment for BCC is surgery, in particular Mohs micrographic surgery (MMS) with safety margins. Topical treatments such as imiquimod, 5-fluorouracil (5-FU), and ingenol mebutate are usually reserved for low-risk cases such as superficial BCC, small BCC (diameter<1cm), or BCC in low-risk areas.1,6 Photodynamic therapy (PDT) is another non-surgical option for low-risk BCC with similar efficacy to topical treatments.
According to the National Comprehensive Cancer Network, BCC can be classified into two categories, low-risk and high-risk (Table 1).
7Low-risk vs high-risk BCC.
| History and physical finding | Low-risk | High-risk |
|---|---|---|
| Location and size | Area L<20mmArea M<10mmArea H<6mm | Area L≥20mmArea L≥10mmArea H≥6mm |
| Borders | Well-defined | Poorly defined |
| Primary vs recurrent | Primary | Recurrent |
| Immunosuppression | (−) | (+) |
| Site of prior radiotherapy | (−) | (+) |
| Pathology | ||
| Subtype | Nodular, superficial | Aggressive growth pattern |
| Perineural involvement | (−) | (+) |
Area H=“mask areas” of face (central face, eyelids, eyebrows, periorbital, nose, lips [cutaneous and vermilion], chin, mandible, preauricular and postauricular skin/sulci, temple, ear), genitalia, hands, and feet; Area M=cheeks, forehead, scalp, neck, and pretibial; Area L=trunk and extremities (excluding pretibia, hands, feet, nail units, and ankles).
Although often localized, in rare cases BCC can progress into a locally advanced (laBCC) or even rarely metastatic BCC (mBCC), especially when left neglected and untreated. There is no established definition of laBCC, but generally, it is considered when the tumor penetrates deeper into the skin as well as surrounding tissues.1,3 Metastasis in BCC is extremely rare, with a rate of only 0.0028–0.55% of all BCC cases. The most common metastatic sites are bones, liver, and lungs.1,3 The median survival rate of patients of mBCC is approximately between 8 months and 7.3 years.1 In cases of laBCC and mBCC, the mutations in the Hh signaling pathway is more prominent compared to the classic BCC, which result in more uncontrolled cell proliferation.8 In these cases, surgical options are not feasible and thus non-surgical approaches should be considered.
Radiotherapy has long been a classic treatment for inoperable BCC especially for people>60 years old.6 Radiotherapy produces considerably good outcome but is less effective compared to MMS.1 However, there are some disadvantages that range from cosmetic, possible development of radiotherapy-induced BCC lesions, and long treatment duration.1 A review article by Dummer, et al determined that surgery and radiotherapy are not suitable in conditions such as: >5 BCCs in patients with genetic syndromes, BCCs with diameter>10mm after 2 surgeries that are located in a critical site such as perioral/periocular region, inoperable BCCs that infiltrate the bone and cartilage, multiple relapses of BCCs after surgery/radiotherapy, or patients with contraindications to general anesthesia.9 Other simple and cost effective techniques in treating BCC include cryotherapy and curettage. These methods are less invasive and can be alternative treatments for BCC. These methods relatively take less resources than radiotherapy or surgery, but its use are limited especially when performed in facial areas and requires expertise as well as well-trained operators to perform in order to prevent complications such as bleeding, tissue damage, and post-inflammatory hypopigmentation.10 This prompted the need to develop more advanced and intricate approaches to deal with inoperable cases.
Research about targeted therapy utilizing systemic treatments such as the Hh signaling pathway inhibitor vismodegib and sonidegib have gained traction over the past few years. Extensive research has been done to explore its efficacy and objective response rate (ORR).8,11 The ORR is based on the widely accepted Response Evaluation Criteria in Solid Tumors (RECIST) criteria which was first established in 2000, and an updated version called the modified RECIST (mRECIST) guideline, mainly to assess tumor changes was published in 2008.12
Hedgehog signaling pathwayHedgehog (Hh) is a series of secreted proteins that act in a signaling pathway between the cell membrane and nucleus called the Hedgehog (Hh) signaling pathway. The term “hedgehog” originated from the protein's first discovery observed in fruit flies (Drosophila melanogaster) during a genetic screen, which showed hair-like-structure in embryos with null alleles resembling the spines of hedgehogs. After its initial discovery, three subsequent types of mammalian Hh ligands were discovered and were named sonic hedgehog (SHH), Indian hedgehog (IHH) and desert hedgehog (DHH).13,14 The Hh pathway plays an integral role during embryogenesis.13,15
These proteins play important roles in early human development. For example, SHH is involved in limb formation, central nervous system development, neural tube development, and formation of lungs, teeth, intestines, and hair follicles, IHH regulates bone and cartilage development, and DHH holds an integral part in germ cell development and peripheral nerve sheath formation.13 After adulthood, the activity of Hh will mostly be reduced, but even then it still takes part in important roles such as homeostasis and wound healing.15
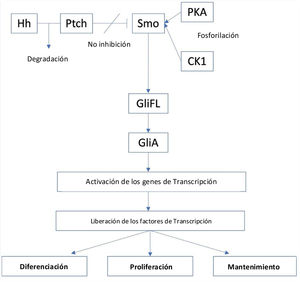
The Hh signaling pathway which starts in the primary cilia, is positively regulated by the membrane protein Smoothened (Smo), a class F G protein-coupled receptor (GPCR), whose activity is regulated by the membrane protein Patched (PTCH).16 There are two types of PTCH receptors, PTCH1 and PTCH2. In the absence of an Hh ligand, PTCH will inhibit Smo activity, and promotes proteolytic cleavage of full-length glioma associated oncogene (GliFL) to Glirepressor (GliR) through phosphorylation by protein kinase a (PKA), glycogen synthase kinase-3 (GSK3) and casein kinase 1 (CK1). GliR will then bind to Hh target gene promoters to prevent activation of Hh target genes, thus turning off the signaling pathway. Conversely, in the presence of Hh ligands, they will bind PTCH and release the inhibition of Smo, initiating the signaling pathway. In addition, Smo is then phosphorylated by PKA and CK1. The signal can then travel downstream via cytoplasmic protein complex comprised of kinesin protein (Kif7), suppressor of fused (SUFU), and GliFL to the tip of the primary cilia. Gli activator (GliA) is then formed and will activate transcription genes. This results in the release of transcription factors such as glioma associated oncogenic factors (Gli) – Gli1, Gli2, and Gli3) that act as the final effector of the pathway that will result in cell differentiation, proliferation, and maintenance (Fig. 1).15,17
Additionally, the role of TP53 gene while not a part of the Hh signaling pathway, can also alter the pathway as its tumor suppressing effect can inhibit Gli transcription. Therefore, the activation of Hh signaling pathway can suppress the activity of TP53 gene.18
The role of Hh signaling pathway in basal cell carcinomaThe aberrant activation of the Hh pathway through mutation in the PTCH1, such as in the loss of function of the gene, will leave no inhibition towards Smo, and the subsequent release of Gli1 and formation of oncogenes as a precursor for BCC. PTCH mutations is a well-known risk factor for recurrent BCC in the autosomal dominant disease of basal cell nervus syndrome (BCNS)/Gorlin's Syndrome. Furthermore, factors such as UVB radiation can also induce PTCH mutation, especially in type I and II skins. Further analysis showed that PTCH mutations were found in 90% of sporadic BCC cases; mutations were found in 9q22.3 locus of PTCH1 (Gene ID: 5727) and 1p34.1 locus of PTCH2 (Gene ID: 8643), making it a suitable target for the development of targeted therapy to treat BCC.17 Other mutations in the pathway, such as in the Smo gene are present in up to a fifth of all BCC cases.15,17 Less commonly, mutations in Smo or TP53 can also lead to BCC formation, as mutations in these genes can also potentially induce Hh signaling pathway reactivation.2,18
Hh pathway inhibitorHh pathway inhibitor (HPI) is a class of biologic drugs indicated for the treatment of inoperable laBCC/mBCC, BCC that often recurs after surgeries or are not suitable for radiotherapy treatment. In addition, HPI can also be used to treat BCNS. To date, two HPIs, vismodegib and sonidegib, have been approved for use in the USA and Europe. Out of these two drugs, vismodegib is the more widely used agent, being already approved for use in more than 60 countries worldwide.8,19
VismodegibVismodegib is a first-in-class HPI which is taken orally with a dosage of 150mg QD. It acts as a PTCH substitute which binds and inhibits Smo, thereby preventing uncontrolled cell proliferation and differentiation.6 Vismodegib can be administered as monotherapy or in combination with other surgical and non-surgical treatment.11 The idea is that the drug will act as a neoadjuvant that can transform previously inoperable BCC cases to become operable. However, this can only be feasible if patients undergo long-term treatment of more than three months with good compliance which could be difficult for some patients due to the adverse effects (AEs).3 There is currently no exact duration of treatment for vismodegib. Patients typically take vismodegib until there is notable improvements or until patients exhibit AEs that merit discontinuation.19
Vismodegib has a high rate of concentration-dependent binding capability to plasma protein (>99%) with a half-life between 4 and 12 days and the volume distribution/Vd (the amount of volume of drug distributed to achieve the same concentration as it is in plasma) of around 27L.20 This suggests that vismodegib is mostly concentrated in the plasma with minimal tissue penetration.9
Adverse effects of the drug, although mostly mild, are one of the key reasons that reduce patient's compliance.21 Some notable AEs are muscle spasms (64%), alopecia (62%), dysgeusia (54%), weight loss (33%), asthenia (28%), decreased appetite (25%), ageusia/hypogeusia (22%), diarrhea (17%), fatigue (16%), and nausea (16%).6 Mild-moderate renal impairment was also examined but further studies are needed to confirm this.22 Vismodegib also showed teratogenic effects and is therefore contraindicated in pregnancy and two years after pregnancy. Male patients are also recommended to have proper contraception when taking vismodegib.11,21,22 Possible hepatoxicity was also reported.11
Alopecia is one of the most common AEs of vismodegib, as inhibiting Hh signaling pathway may also affect hair follicle formation. Fortunately, the AE is reversible with patients reporting improvements after 6–12 months of treatment discontinuation.11 In a case report by Villani, et al, the use of topical minoxidil can help reduce diffuse hair loss.23 Dysgeusia or loss of taste buds is also attributed to vismodegib as Hh signaling pathway affects formation and maintenance of taste buds, that can lead to subsequent loss of appetite, weight loss, and ultimately depression.11 Interestingly, a recent review reported that the incidence of AEs in patients on treatment for more than 12 months are significantly lower compared to in the first 12 months of treatment.22
In terms of drug interactions, according to the EU summary of product characteristics, vismodegib concentration decreases when co-administered with CYP-inducers such as carbamazepine, rifampicin, and phenytoin.22 Vismodegib is also not recommended to be taken together with drugs that alter hepatic metabolism.11
Information about resistance to vismodegib is still scarce. There are a few case reports such as by Rudin et, al on a 26-year-old male patient with medulloblastoma that initially showed tumor regression after 3 months of 150mg QD treatment. However, loss of efficacy was noted after three months of treatment. It was hypothesized that this resistance was due to Smo mutations that developed during treatment. Another study found that a heterozygous missense mutation at position 1497 of Smo caused the switching of aspartic acid (Asp) for histidine (His) in codon 473 (D473H). Mutations in Smo causing less effectiveness of vismodegib's capability to bind to Smo was also mentioned by Djikgraaf, et al. They found 21 mutations in the Smo region, in particular Smo-E518A which showed the most prominent spike in activity.24
The initial phase-1 clinical trial of vismodegib was first reported in 2009 by von Hoff et al., with three different doses of 150mg, 270mg, and 540mg QD.11,25 A follow-up study in 2011 by Loruso, et al. found that the optimal dosage for vismodegib was 150mg QD, since higher dose did not increase plasma concentration. The approval of vismodegib use by the EU and USA was based on two important clinical trials by ERIVANCE and STEVIE.4,11,22
ERIVANCE was a central review-assessed, single-arm, two-cohort, international multicenter phase-2 clinical trial done on 104 patients (33 mBCC patients and 71 laBCC patients) receiving 150mg QD oral vismodegib. Initially the ORR was 30% and 43% ORR, respectively. However, a follow-up study after 30 months found increased ORR to 60% in laBCC and 48.5% in mBCC with a median duration of treatment of 26.2 months for mBCC and 14.8 months for laBCC.6 The study concluded that with increased treatment duration, there was increase in overall ORR.8 Disease-free survival times were 12.9 months and 9.3 months respectively.6 The final update of this trial was reported in 2017, with a total trial period of 39 months and overall ORR was 60.3% for laBCC and 48.5% for mBCC.8 A total of 33 patients died with the most common cause being disease progression and adverse effects.8 Average progression-free survival (PFS) or the amount of time during and after treatment in which patients do not experience progression of disease was 12.9 months for laBCC and 9.3 months for mBCC. Duration of response (DOR) for laBCC averaged around 26.2 months for laBCC and 14.8 months for mBCC.11
Another study, STEVIE, a single-arm, two-cohort, non-randomized (non-comparative) open-label study, is to date the largest number of participants to date for vismodegib. It involved 1215 patients consisted of 1119 laBCC (208 BCNS patents) and 96 mBCC patients for a total of 86 months. Primary analysis found that the ORR of laBCC was 68.5% and mBCC was 36.9%. Similarly, in a phase-2 clinical trial, 38% ORR was observed in mBCC.2,19 PFS was 23.2 months for laBCC and 13.1 months for mBCC. Furthermore, DOR was 23.0 months for laBCC and 13.9 months for mBCC.11
A review by Brancaccio et al. compiled a 21-month analysis on the efficacy of vismodegib to treat laBCC. They found that the mean percentage of ORR was 47.6%, with 25.4% showed partial response rate while 22.2% achieved complete response. Additionally, 34.9% of patients were in stable condition after consuming vismodegib and 12.7% experienced progression.20 A study by Chang, et al found similar result for vismodegib ORR, with 46.4% for laBCC and 30.8% for mBCC. However, the PFS and DOR were unable to be assessed due to the limited follow-up period.11
Recently, a study suggested that GAS-1, one of the 40 Hh signaling pathway genes, showed the most prominent decrease in expression after vismodegib treatment. Hence, quantifying GAS-1 has emerged as a potential indicator for treatment response. However, since this study was done with a relatively small sample size (22 patients), further studies in this field are needed.26
The latest study regarding vismodegib was published in 2021 in the form of VISMONEO study, where it studied the efficacy and safety of vismodegib as a neoadjuvant treatment in laBCC in an open-label phase 2 trial. Vismodegib was administered orally at a dose of 150mg for 4–10 months in both operable and inoperable laBCC. A total of 55 patients with the median age of 73 years old. Overall response rate according to the RECIST criteria at 71%. (95% CI). Vismodegib was also found to promote downstaging of laBCC especially in sites with great functional use.27
SonidegibAfter the initial success of vismodegib, another oral HPI with similar attributes was developed. Sonidegib has similar mechanism of action as vismodegib.28 The drug is already approved by the FDA and EMA in 2015.20,29 The drug acts by binding to the Smo protein thus suppressing Gli proteins, and subsequent proliferation and tumor growth.29
To date, head-to-head data of vismodegib and sonidegib is yet to available.9 Furthermore, previous studies on vismodegib and sonidegib used different interpretations in determining ORR. The ERIVANCE study of vismodegib used the RECIST criteria, while sonidegib used the mRECIST criteria.9,20 Sonidegib possesses different and interesting pharmacokinetics as compared to vismodegib. The first difference is concentration dependance. While vismodegib and sonidegib both have high binding capabilities (>99%) to plasma proteins alpha -1-acid cycloproteins (AAG) and human serum albumin (HSA), the binding capability in sonidegib is non-concentration-dependent, while vismodegib is concentration-dependent.9,20 Additionally, sonidegib's Vd is significantly higher (9170L) compared to vismodegib (27L). This may also explain why the concentration of sonidegib on the skin is six times higher compared to when in plasma, and half-life of sonidegib is 28 days as compared to only 4 days for vismodegib.9,20 These differences prompt the possibility of efficacy discrepancies between the two drugs.
In terms of drug interactions, it is recommended to not co-administer sonidegib with CYP3A4 inhibitors, as sonidegib is a substrate of CYP3A4. Conversely, CYP3A4 inducers should also be avoided, or alternatively the dosage of sonidegib can be increased to 400–800mg.20 A phase-1 study also found that the absorption of sonidegib is reduced when co-administered with 40mg of esomeprazole.20 The drug is metabolized in the liver via oxidation and amide hydrolysis involving CYP enzyme.28
Reports of resistance towards sonidegib are still very limited; an open-label trial on laBCC showed that patients resistant to vismodegib that were treated with sonidegidib also experienced resistance to sonidegib, with five out of nine patients exhibiting Smo mutations. The mutations sites differed between patients; reported sites included Q477, D473 (2 patients), S533, and W535. These mutations resulted in the inability of sonidegib to bind to Smo. However, it should be noted that the small sample size underwent only short median duration of treatment (5 weeks) due to premature discontinuation related to adverse effects.30
One of the pivotal trials that led to the approval of sonidegib was the BOLT trial, a multicenter, randomized, double-blinded phase-1 trial conducted in 2014 that was assessed by a central review. During this study, it was found that the maximum tolerable dosage for the drug was 800mg or 2×500mg daily. The study also added that 200mg was the optimal dosage with consideration in the bioavailability, efficacy, and AEs.29 A long-term 42-month study found similar efficacy to vismodegib with an ORR of 56.1% and 46.1% in laBCC patients receiving 200mg and 800mg dosage, respectively.29 However, for mBCC, 800mg dosage was found to produce higher ORR (17.4%) compared to 200mg dosage (7.7%).29 Additionally, the LDE225 trial, a multicenter randomized double-blind phase-2 study found that 200mg sonidegib produced better ORR towards laBCC with 43% compared to 38% in patients on 800mg sonidegib, while 800mg was more superior for mBCC with 17% ORR compared to 15% in 200mg dose.20 Furthermore, this study also found the potential use of oral 800mg sonidegib to treat BCNS. In a small study of 13 BCNS patients, 8 experienced complete response. Topical sonidegib with 0.75% concentration was also explored as a mean to treat BCNS, with 3 out of 13 patients experienced complete response. More trials are needed, but initial results showed that sonidegib is promising to treat BCNS.20,28,29
A review by Brancaccio, et al stated the ORR based on RECIST criteria of sonidegib after an 18-month period of laBCC treatment was 60.6%, in which 21.2% achieved complete response and 39.4% patients achieved partial response. Patients with stable disease was at 30.3%, while 1.5% of patients experienced disease progression.20
As with vismodegib, patient compliance is an important factor impacting efficacy. Compliance in patients with a lower dose is significantly higher, with almost a 30% difference compared to patients on higher dose. Average compliance was 8.9 months for 200mg sonidegib and 6.5 months in 800mg dosage.6 Furthermore, lower dosage is attributed to less AEs, with similar AEs as in vismodegib. Muscle spasm is the most common AE (67%) in patients on 800mg sonidegib; the incidence was lower (49%) in patients on 200mg sonidegib. Alopecia and dysgeusia are the second and third most common AE (43% and 38%), but the rate is still lower compared to vismodegib (62% and 54%). Interestingly, ageusia/hypogeusia was not found in patients on 200mg sonidegib, while almost a fifth of patients on 150mg vismodegib experienced them.6,28 In addition, a small number of participants on 200mg sonidegib experienced more serious AEs such as hypertension (3%), elevated creatine kinase (3–4%), and increased lipase (5%).29 Lastly, pregnant patients are contraindicated for sonidegib as it is classified as a class-D drug that is proven to be teratogenic and can cause fetal death.28 Thorough screening such as complete blood count and renal function, as well as sufficient patient education are needed prior to administration. If AEs persist, treatment should be halted.
The most recent study to combat adverse events is a limited study by Villani, et al and published in 2021. The aim of the single center study was to evaluate whether dosage reduction of sonidegib would reduce the AEs experienced. 9 patients received daily 200mg sonidegib treatment between 12 and 24 weeks, followed with dosage adjustment of 1 dose every second day for 8–16 weeks. The study found that patients experienced less severity of AEs hence increasing patient's tolerability for the long treatment of sonidegib. This prompt the need for further research to confirm this method.31
Role of HPI in BCC therapySeveral BCC guidelines worldwide have approved the use of HPI to treat BCC. This includes the National Comprehensive Cancer Network (NCCN), the American Academy of Dermatology (AAD), and the European Association of Dermato-Oncology (EADO).
NCCN Guideline in 2016 are still considering the use of systemic therapy such as HPI to treat metastatic BCC, as they were still on clinical trial, and a revised 2021 guideline are currently in the making.7 In addition the AAD Guideline In 2017 have recommended a multidisciplinary approach to treat metastatic BCC. The use of HPI should be considered in cases where radiotherapy is contraindicated in patients, and if there are residual tumors following surgery and/or radiotherapy, as well if patients are inoperable.32 Similar with the two previous guidelines, the EADO Guideline has also recommend the use of HPI to treat “difficult-to-treat” BCC such as laBCC, mBCC, and Gorlin Syndrome.33
Role of HPI as neoadjuvant therapy for BCCAlthough initially approved as the primary therapy for inoperable laBCC, and mBCC, there are also studies for its purpose as neoadjuvant therapy to reduce extensive surgeries as well as to increase success of surgeries. Patients are treated with HPIs until there are no further response and then undergone surgeries, this method can significantly promote both complete and partial response as demonstrated in studies by Ching et al. and Wong et al.37,38 The first study found that the use of vismodegib as a neoadjuvant therapy can avoid bone resection in 50% of subjects, and the latter study on patients with ocular and orbital BCC achieved 67% complete response and a notable patient was able to have orbital salvation.34,35,37,38 The EADO Guideline also recommends its use as a neoadjuvant to treat laBCC. However, there are yet to be a randomized data available to proof its efficacy. Although an initial study of 15 patients showed satisfactory result, where only one patient suffered recurrency after 22 months post-surgery after consuming vismodegib for 3–6 months.33
ConclusionThe HPIs vismodegib and sonidegib are novel treatments for inoperable laBCC and mBCC and is a promising treatment for BCNS. Both drugs produce overall great response and can be used as either monotherapy, combination with other therapies, or as neoadjuvant therapy for BCC. AEs of HPI, although mild, are often, and can influence patient compliance. Further studies are needed to find possible efficacy differences between the two drugs as there has yet a head-to-head study with similar assessment criteria.
Conflict of interestThe authors declared no conflict of interest.