Alopecia areata is an autoimmune disease that affects the hair follicle and can present as bald patches on the scalp and hair loss in other parts of the body. Diagnosis is clinical but can be aided by trichoscopy, a simple, rapid technique that reduces the need for invasive procedures and can also help with monitoring treatment response. We review the usefulness of trichoscopy in alopecia areata. The most common trichoscopic findings are yellow dots, black dots, exclamation mark hairs, short vellus hairs, and coudability hairs. Other, less common, findings can also help establish a diagnosis. Good response to treatment is indicated by the disappearance of black dots, broken hairs, and exclamation mark hairs. The observation of yellow dots, by contrast, indicates chronic disease and poor response to treatment.
La alopecia areata es una enfermedad autoinmune que afecta al folículo piloso. Se presenta en forma de placas alopécicas e incluso pérdida de pelo corporal. El diagnóstico es clínico. Sin embargo, la tricoscopia, una técnica valiosa no invasiva, simple y rápida, mejora el diagnóstico, la monitorización del tratamiento y reduce la necesidad de procedimientos invasivos. Realizaremos una descripción de la utilidad de la tricoscopia en la alopecia areata. Los hallazgos tricoscópicos más frecuentes de la alopecia areata son los puntos amarillos, los puntos negros, pelos en signo de exclamación, pelos vellosos cortos y pelos acodados. Sin embargo, existen otros hallazgos menos frecuentes pero útiles para realizar el diagnóstico. La buena respuesta al tratamiento implica la desaparición de los puntos negros, los pelos rotos y los pelos en signo de exclamación, pero la presencia de los puntos amarillos indica enfermedad crónica y mala respuesta.
Alopecia areata (AA) is a type of autoimmune noncicatricial alopecia. Its prevalence is 0.1–0.2% in the general population, and the lifetime risk of having the disease is estimated to be 1.7–3.8%. The disease becomes chronic in 8% of affected patients, with no differences according to race, sex, or age.1–3 Familial incidence ranges from 10% to 20%,1,2,4 and the disease is associated with other autoimmune conditions in 30% of cases.1,2,4
AA affects the scalp in 90% of patients, although it can appear in other hairy areas such as the eyelids, eyebrows, and beard, as well as the nails. It can appear as a single or various well-delimited patches, and with diffuse or complete hair loss. Its course is variable and unpredictable. No cure is known.1,2,4
Diagnosis is based on clinical findings and includes the patient's clinical history and a physical examination. Evaluation of disease activity and severity at the first visit can provide important prognostic information on the disease and its treatment. Clinical examination with trichoscopy is useful.1,2,4
Trichoscopy refers to the application of dermoscopy on the scalp and hair. This useful technique is simple, noninvasive, and easy to learn and reduces the need for more aggressive procedures such as biopsy. Use of dermoscopy in the dermatology clinic can improve diagnosis of AA, help to evaluate inflammatory activity, identify signs of severity, assess therapeutic control, and reveal prognostic factors. It is considered an essential additional technique in the trichology clinic.2,4,5
The primary objective of this study was to review the most common findings in trichoscopy in AA to date and to determine their association with inflammatory activity, severity, and prognosis.
Trichoscopy Findings in Alopecia AreataYellow dotsYellow dots are dilated infundibula filled with sebum.4,6,7 They are regularly distributed in groups of 1, 2, or 3, thus reflecting the number of shafts per follicular unit.8 They vary in color, shape, and size and may manifest as yellow, yellow-pink, yellow-brown, whitish, round, or polycyclic dots or as dots with a double border and uniform color. Hair may be absent or miniaturized, cadaver, or dystrophic.6,9,10
The frequency of yellow dots varies with age, Fitzpatrick skin phototype, imaging technique applied (polarized or nonpolarized light dermoscopy and use of immersion oil), and the patient's shampooing habits.9 Yellow dots are less visible on yellow or dark skin (phototypes IV–V).8,10 In the latter case, empty follicles may appear white instead of yellow, with the result that the follicle opening must be differentiated from eccrine ducts.8,11
Yellow dots are present in 60%–94% of cases of AA.12–14 They are less frequent in children because the sebaceous glands are not completely developed before puberty.15,16
Yellow dots are a marker of severity, since their frequency increases in chronic forms of AA, where they are very numerous and regularly distributed.2,17
This trichoscopy finding is highly sensitive for diagnosis of AA, although it is not very specific.14,16 It may be present, albeit to a lesser extent, in other types of alopecia, such as discoid lupus erythematosus, androgenetic alopecia, trichotillomania, chronic telogen effluvium, tractional alopecia, psoriasis, kerion, and congenital hypotrichosis10,17,18 (Fig. 1).
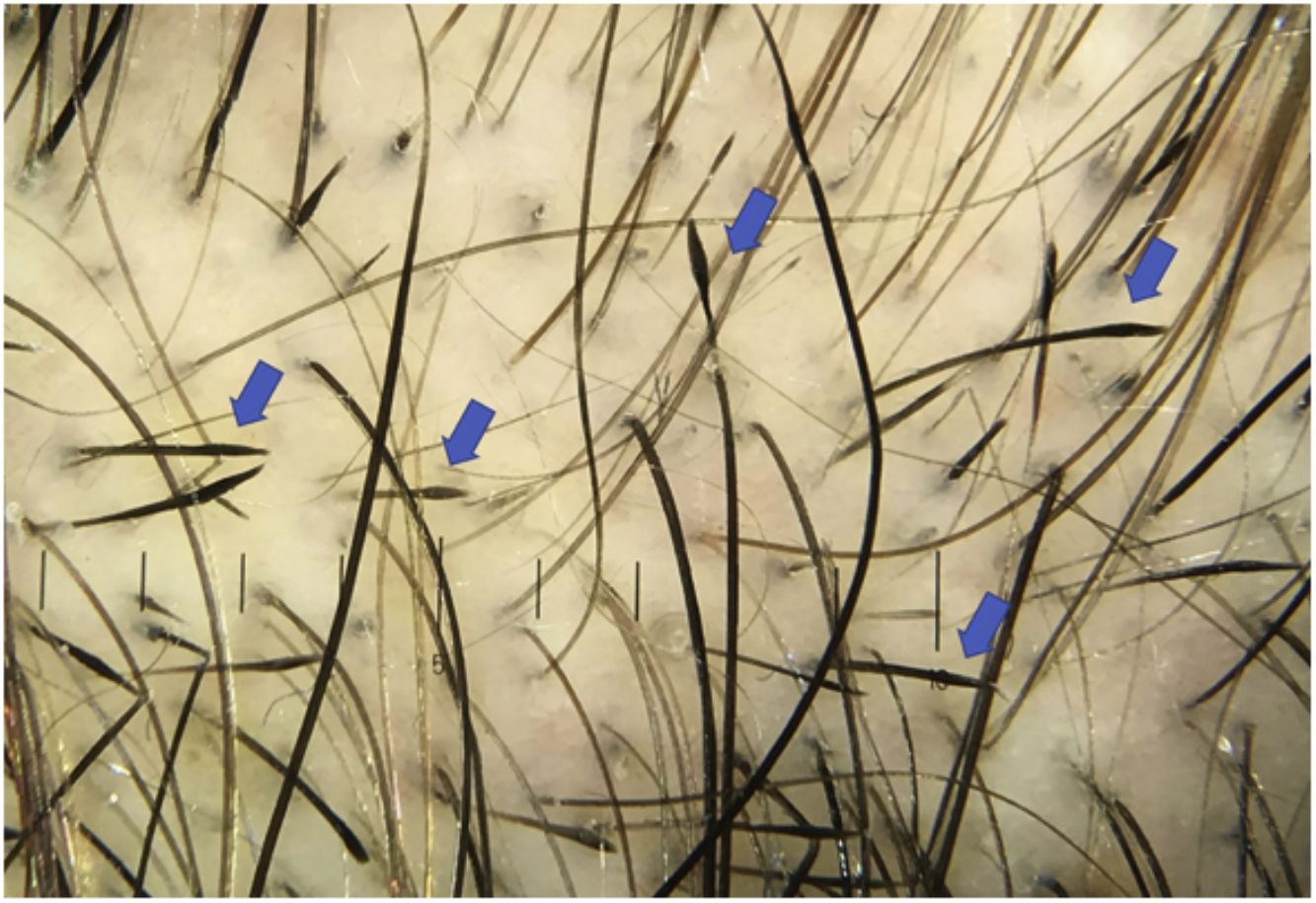
Black dotsBlack dots are pigmented hairs broken at the level of the scalp, where the root remains joined to the follicle opening, somewhat resembling a comedo.4,7,8 They correspond to inflammatory damage to the hair follicle leading to abrupt cessation of mitotic activity and weakening of the proximal portion of the hair shaft. Hence, the hair breaks as soon as it leaves the follicle opening.19,20
Black dots vary in size, and 2 or 3 dots may be grouped together, thus demonstrating that all the hair shafts in a follicular unit are affected by the disease. Black dots are slightly larger than the thickness of the terminal hair shafts in the anatomical region where they are found.8
Black dots are observed in 50% of cases of AA,2 although case series report wide variations in frequency (e.g., 0%–84%,4 44%–70%8). The presence of black dots is affected by the skin phototype of the study population, since the hair color and cuticle also change. Black dots are found more frequently in patients with skin phototypes III-IV than in Whites (mean, 57%–63% vs. 49%).18
In children, black dots should be differentiated from the rectangular black granular structures that are characteristic of loose anagen syndrome.4
Black dots are characteristic of AA and are indicative of active disease. When found in larger numbers, they point to a poor prognosis.13,14,17 They are also considered a negative predictive marker of response to treatment.21 However, they are not considered a specific sign,4,8 since they may be present in other diseases, such as trichotillomania (main differential diagnosis),13,19 tinea, dissecting cellulitis of the scalp, chemotherapy-induced alopecia, lichen planopilaris, discoid lupus erythematosus, traction alopecia, cutis aplasia, androgenetic alopecia, and alopecia occurring after laser hair removal or after a trichogram.1,14,16 Topical immunotherapy combined with anthralin stains the follicle openings, leading them to resemble black dots19 (Fig. 2).
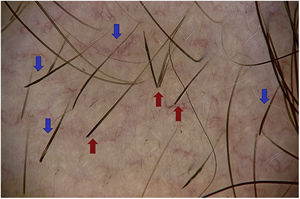
Exclamation mark hairsExclamation mark hairs are broken hairs with a fine, hypopigmented proximal end and a thicker, hyperpigmented distal end.1,16,22 They may result from a transient cellular degeneration phase between a precortical keratinocyte phase and defective cortical differentiation.23,24
Exclamation mark hairs are observed at the edge of active lesions,14,17,20 both to the naked eye and in trichoscopy; the former measure 1–3cm in length and the latter measure 1–5mm.6 Optical microscopy shows the distal ends of these stems to be irregular (frayed) in 77.8%.23
The prevalence of this finding in AA ranges between 12% and 71%, with a mean of 39%.24 Exclamation mark hairs are found mainly in the acute, progressive form and in the stable phase of AA.18,23 They indicate the activity and severity of the disease and are a negative prognostic marker.21 Their presence could be a positive predictive marker of the response to corticosteroids.24
Exclamation mark hairs are characteristic of AA. However, they can also be found in trichotillomania, traction alopecia, tinea capitis, chemotherapy-induced alopecia, anagen effluvium caused by poisoning, and, to a lesser extent, in androgenetic alopecia4,14 (Fig. 3).
Tapered hairsTapered hairs are very similar to exclamation mark hairs, with a finer proximal end and a thicker distal end. However, the difference is that they are longer, to the extent that they fall outside the visual field of the dermoscope.24 Tapered hairs are terminal hairs that are normal in length.4 They are visible to the naked eye and are usually found around lesions. Tapered hairs are indicative of activity and are considered negative predictive markers of hair regrowth.21 They appear during the early stages of the disease and precede the appearance of black dots and exclamation mark hairs.2,6
According to various studies, the frequency of tapered hairs in AA ranges from 5% to 81%, with a mean of 51%. Moreover, they are observed in patients with trichotillomania, malnutrition, chemotherapy-induced alopecia, anemia, and chronic poisoning4 (Fig. 4).
Coudability hairsThe term “coudability hairs” derives from the similarity of the hairs to a coudé catheter. Coudability hairs bend easily when pressure is applied toward the interior of the scalp, leading to a twist in the axis, where damage and narrowing of the hair shaft occur. The proximal end is thinner, and the length is normal. The proximal narrowing of the hair shaft could be due to a rapid anagen-to-catagen transition, thus highlighting a pathological adaptability mechanism. The kinked hairs represent a less severe injury of the hair follicle, which would lead to dystrophic anagen and narrowing of the proximal shaft.25 Couldability hairs have been associated with the inflammatory activity of the disease17,26 and are a pathognomonic sign of AA.17,18
Broken hairsBroken hairs are caused by a cross-sectional fracture of a previously weakened hair shaft. They may also result from rapid growth of partially destroyed hairs, which are seen as black dots. In contrast with exclamation mark hairs, which have a fine and hypopigmented end, broken hairs are short, with a hair shaft that is normal in appearance, except at the distal end, where it is irregular and torn.24
Broken hairs predominate in acute AA4,9,18 and are negative prognostic markers.21 They are observed in 0%–71% of cases of AA (mean, 49%),4,18 as well as in trichotillomania,1,4 tinea capitis, traction alopecia (traumatic), primary cicatricial alopecia, androgenetic alopecia, and telogen effluvium.8
The camouflage products used by patients, such as hair thickening fibers, can be confused with broken hairs19 (Fig. 3).
Vellus hairsVellus hairs have a pigmented proximal end and the same thickness along the shaft. They are indicative of regrowth, spontaneous disease remission, and favorable response to treatment. They vary in length depending on disease stage.12,20,24
Vellus hairs are found in 34%–100% of cases and are more frequent in patients with light skin phototypes (48%–100%) than in those with dark phototypes (34%–94%).6 They are the most common trichoscopy sign in long-term AA.12,24
Vellus hairs are a poorly specific sign. They are reported in trichotillomania, tinea capitis, traction alopecia, traumatic alopecia, congenital triangular alopecia, telogen effluvium (chronic and acute), and primary cicatricial alopecia.2,4,24
Upright regrowing hairsRegrowing hairs are new, healthy hairs that grow vertically. They have a thick, pigmented proximal end and a tapered distal end. They are usually upright or slightly bent.2,6 These hairs are a marker of regrowth and a positive predictive therapeutic marker.21
Upright regrowing hairs are seen more often in children, probably because more extensive growth of the hair is more common at this age. They are not pathognomonic of AA and are observed in acute telogen effluvium, trichotillomania, tinea capitis, and temporal triangular alopecia.4,24
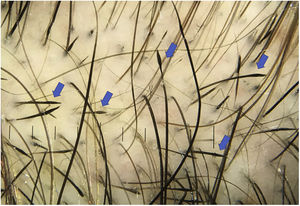
Pigtail (circle) hairsPigtail hairs are short, regularly curled regrowing hairs that take on a circular or oval shape. They are considered a positive predictor of regrowth in patients with acute or diffuse AA after treatment or in patients who achieve spontaneous remission.8,11,21
Pigtail hairs are uncommon and more frequently found in children.15,24 Their prevalence varies according to the study, ranging from 4% to 61% (mean, 21%).14,15
Pigtail hairs are not a specific sign, as they can also be seen in chemotherapy-induced alopecia, at the edge of cicatricial alopecia, in tinea capitis, in trichotillomania, and in temporal triangular alopecia (Fig. 5).14,24
Pohl-Pinkus constrictionsThe term Pohl-Pinkus constrictions was coined by the German dermatologist Joseph Pohl-Pinkus in 1885. It refers to narrowing of a hair along the length of the shaft. These constrictions are due to rapid and sudden suppression of metabolic and mitotic activity in the follicle caused by both external and internal factors. Therefore, Pohl-Pinkus constrictions are the point where the lesion occurs (several constrictions can be observed in recurrent episodes). Narrowing is progressive and irregular along the shaft.8,24,27 It is uncommon in AA (2%–10%).27
Pohl-Pinkus constrictions are not exclusive to AA and can also be seen in chemotherapy-induced alopecia, cicatricial alopecia, after severe infections, after major blood loss, in nutritional deficiencies, after therapy with interferon, and in localized hereditary hypotrichosis24,27 (Fig. 6).
Other Trichoscopy Findings in Alopecia AreataOther interesting trichoscopy findings have been reported.
Honeycomb patternHoneycomb pattern is a reticular structure comprising multiple rings that are between yellow and brown in color. The rings are arranged homogeneously side-by-side or mosaic-fashion in areas chronically exposed to direct sunlight and lacking in hair. Honeycomb pattern has been observed in 13% of cases and is considered a marker of chronic disease.1,4,8
Dirty dotsDirty dots are nonmicrobial environmental particles that disappear after shampooing. They can be brown, black, red, yellow, or blue and can appear as loose fibers.28
Dirty dots are more common in children and elderly people and are considered to mimic black dots.4,29
White dotsThe 2 types of white dots are large white dots and small pinpoint white dots.4,11
Large white dots, which are present in chronic AA, are irregular and correspond to areas of follicular fibrosis. Pinpoint white dots are regular, smaller, and correspond to the openings of the eccrine sweat glands. They can be found in sun-exposed areas and in dark skin phototypes.1,4,17
Tulip hairsTulip hairs differ from exclamation mark hairs in that they narrow slightly with diminished coloring at their proximal end and have a darker distal end, where the hair fractures. Incidence ranges from 2% to 10%.5,6 They are not characteristic of AA and can be observed in trichotillomania8 (Fig. 7).
Usefulness of Trichoscopy for Monitoring of Treatment and Application of Trichoscopy Findings in Alopecia AreataThe trichoscopy findings set out above are directly related to various aspects of AA. Some are associated with the inflammatory activity of the disease, duration of alopecia, severity of the clinical picture, clinical presentation, and response to treatment.
Inflammatory activity in AA: Inflammatory activity determines whether the hair is being attacked by an inflammatory infiltrate that is leading to hair loss and impeding regrowth. The main trichoscopy findings are summarized in Table 1.
Trichoscopy Findings in Alopecia Areata: Association With Disease Activity, Severity, and Duration.
| Signs of activitya | Yellow dots2,8,14Exclamation mark hairs4,18,23Broken hairs8–10 Tapered hairs2,6,23Pohl-Pinkus constrictions23,27Coudability hairs3,25 |
| Signs of inactive disease | Yellow dots2,4,18Vellus hair2,4,18Empty follicular openings17White dots17 |
| Signs of regrowth | Upright regrowing hairsPigtail hairsVellus hairs2,4 |
| By severity (more severe) | Yellow dotsBlack dotsReduced vellus hairsHoneycomb hyperpigmentationWhite dotsCumulus-like clustered white dots17 |
| By severity (less severe) | Exclamation mark hairs3,4 |
| By disease duration (acute) | Exclamation mark hairsBlack dotsVellus hairs12 |
| By disease duration (chronic) | Smooth and fine scalp hairPresence of follicular openings that are obstructed by keratotic plugs known as yellow dots12Scant regrowth of pigmented hairs and homogeneous distribution8 |
While different forms are observed, the manifestations are similar to the response of the hair follicle to the inflammatory process in the area of the hair bulb.3,11
Duration: AA can be defined as acute, when hair loss in a specific area is gradual with positive results in the traction test and trichogram, and chronic, when hair loss in a specific area is long-term and persistent without regrowth (Table 1).
Severity: Severity is classed according to hair loss and is considered an important prognostic factor. Clinical evaluation should be based on the Severity of Alopecia Tool, which scores the percentage of hair follicles lost (Table 1).
Clinical: AA has several forms of presentation: as a single patch, various patches (multifocal), total (loss of all the hair on the scalp), universal (loss of hair on the scalp and the rest of the body), and diffuse or incognita (characterized by rapid and diffuse thinning and increased loss). It can also be classified as ophiasic, when hair loss occurs along the frontal–parietal–occipital hairline (Table 2).
Alopecia Areata (AA): Clinical Type and Trichoscopy Findings.
| Clinical type | Trichoscopy findings |
|---|---|
| Alopecia areata incognita (AAI) | Pigtail hairs (57.9%)Regrowing hairsBlack dotsDiffuse yellow dots33 affecting mainly the occipital and parietal areas and proportional to severity13 |
| Diffuse alopecia areata | Follicular inflammatory damage is much greater than in AAIGreater presence of dystrophic hairsBlack dots (36%)Yellow dots in the parietal and anterior-temporal areas of the scalp13 |
| Alopecia incognita | Yellow dotsShort vellus hairs, 96% sensitivity |
| AA with ophiasic pattern | Black dotsAbsence of yellow dots34,35 |
| AA total and universal | Positive predictive markers:Pigmented vellus hairsUpright regrowing hairsPredictive markers that are not useful for evaluating the response to treatment:Black dotsBroken hairsExclamation mark hairsTapered hairsPohl-Pinkus constrictions36 |
Predictors: Predictors can be positive or negative. Positive predictors indicate a greater probability of regrowth and can be observed in trichoscopy (Table 2).
As mentioned above, trichoscopy plays a key role in monitoring of treatment in AA.
Patients who receive topical treatment with local UV-A phototherapy and methoxypsoralen gel combined with minoxidil 5% solution have been shown to have “hidden hairs” in the proximal part of the hair follicle17 and peripilar sign as an indication of growth.20
Treatment with intralesional triamcinolone acetonide has been shown to lead to regrowth of nonpigmented vellus hairs and the transformation of vellus hair to terminal hair. In addition, exclamation mark hairs, broken hairs, and black dots disappeared earlier than yellow dots.7
Platelet-rich plasma (PRP) and intralesional corticosteroids were compared in AA in patches. Reductions were recorded in both groups in the number of broken hairs, exclamation mark hairs, tapered hairs, black dots, and upright regrowing hairs, whereas no significant changes were observed for yellow dots or vellus hairs.30
The efficacy of PRP was compared with that of topical minoxidil 5%. The authors reported an increase in the number of short regrowing hairs and a decrease in the number of yellow spots with minoxidil solution, whereas with PRP, the number of pigmented hairs increased and that of vellus hairs and yellow dots decreased. In addition, the response was earlier and more effective.31
One research group developed a scoring system for patients with rapidly progressive AA in order to predict the outcome of treatment with intravenous corticosteroid pulses. The system was based on statistical correlations using analysis of digital trichoscopy images.32
ConclusionsTrichoscopy is a simple, fast, noninvasive, and indispensable technique in the dermatology clinic. It can improve diagnosis, evaluate disease activity and severity, and enables early and better therapeutic control.
The most frequent trichoscopy findings in AA are yellow dots, black dots, broken hairs, exclamation mark hairs, tapered hairs, coudability hairs, and vellus hairs.
Trichoscopy findings have been associated with disease activity, potential response to treatment, severity and duration of alopecia, and the patient's clinical condition. Knowledge of these findings can help the dermatologist to tailor treatment of AA.
Conflict of interestThe authors declare that they have no conflicts of interest.