Mycobacterium chelonae is an atypical mycobacterium classified as a rapidly growing nonchromogenic mycobacterium.1 It is universally distributed and is normally found in the environment (e.g., in water and soil).2–5 It is one of the most common mycobacteria responsible for skin infections in immunocompromised patients in whom lesions may be deeper and/or more disseminated. Infections can manifest as abscesses, painful erythematous nodules,2 folliculitis, cellulitis, and sporotrichoid lesions.3 Most cases are nosocomial and are generally associated with trauma or surgical or cosmetic procedures, although these events are often not evident.2–5
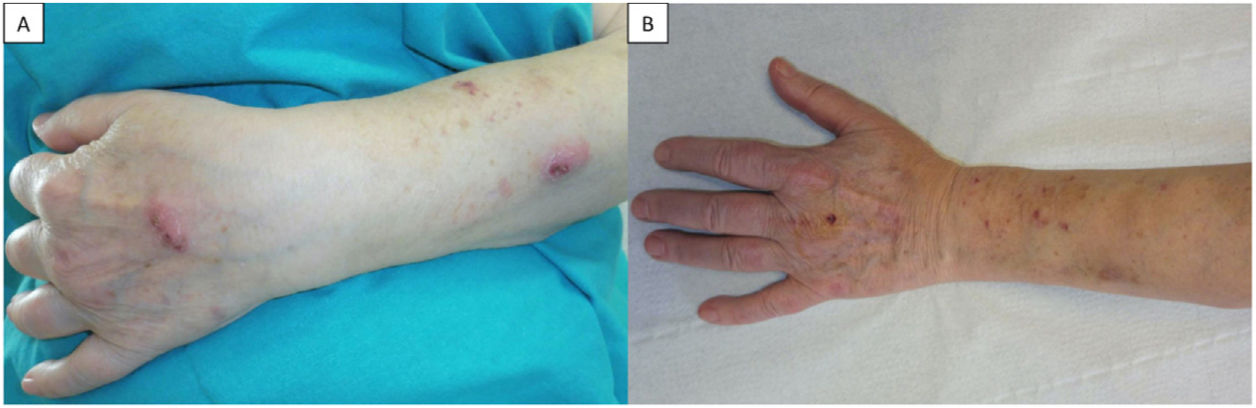
A 70-year-old woman with stage III sarcoidosis under treatment with salmeterol/fluticasone propionate, terbutaline, and inhaled prednisone (10mg daily) presented at the dermatology clinic with lesions of 1 month's duration on her left forearm. She did not recall any previous trauma and reported no fever or associated systemic symptoms. Physical examination showed 2 erythematous nodules, firm to palpation, with sporotrichoid spread: one on the dorsum of the left hand and the other on the dorsum of the left forearm, (Fig. 1A). Suspecting deep mycosis or cutaneous sarcoidosis, we performed skin biopsy, which showed an intense inflammatory infiltrate in the deep dermis composed of lymphocytes, histiocytes, and clusters of polymorphonuclear leukocytes with cell debris. Ziehl–Neelsen staining showed long pink structures (Fig. 2). With a histopathologic diagnosis of suppurative granulomatous nodular dermatitis of probable infectious origin, DNA was extracted for mycobacterial species identification by polymerase chain reaction (PCR), which, together with the culture findings, confirmed a diagnosis of skin infection due to M. chelonae. The patient was prescribed clarithromycin 500mg/12h for 4 months. She responded well initially, but on completion of treatment, she developed a recurrent infection. Susceptibility testing at this point showed susceptibility to clarithromycin, ethionamide, and tobramycin. Follow-up tests revealed hypogammaglobulinemia with an immunoglobulin (Ig) G level of 380mg/dL (normal, >650mg/dL) and B-cell lymphopenia (30cells/mL; normal, >100). On reviewing the patient's clinical records, we detected a history of respiratory infections and bronchiectasias and established a diagnosis of a primary immunodeficiency disorder (PID) with predominantly deficient antibody production. The patient was started on intravenous IG replacement therapy at a dose of 0.4mg/kg every 3 weeks, which, together with clarithromycin for 2 months, led to definitive resolution of the lesions (Fig. 1B).
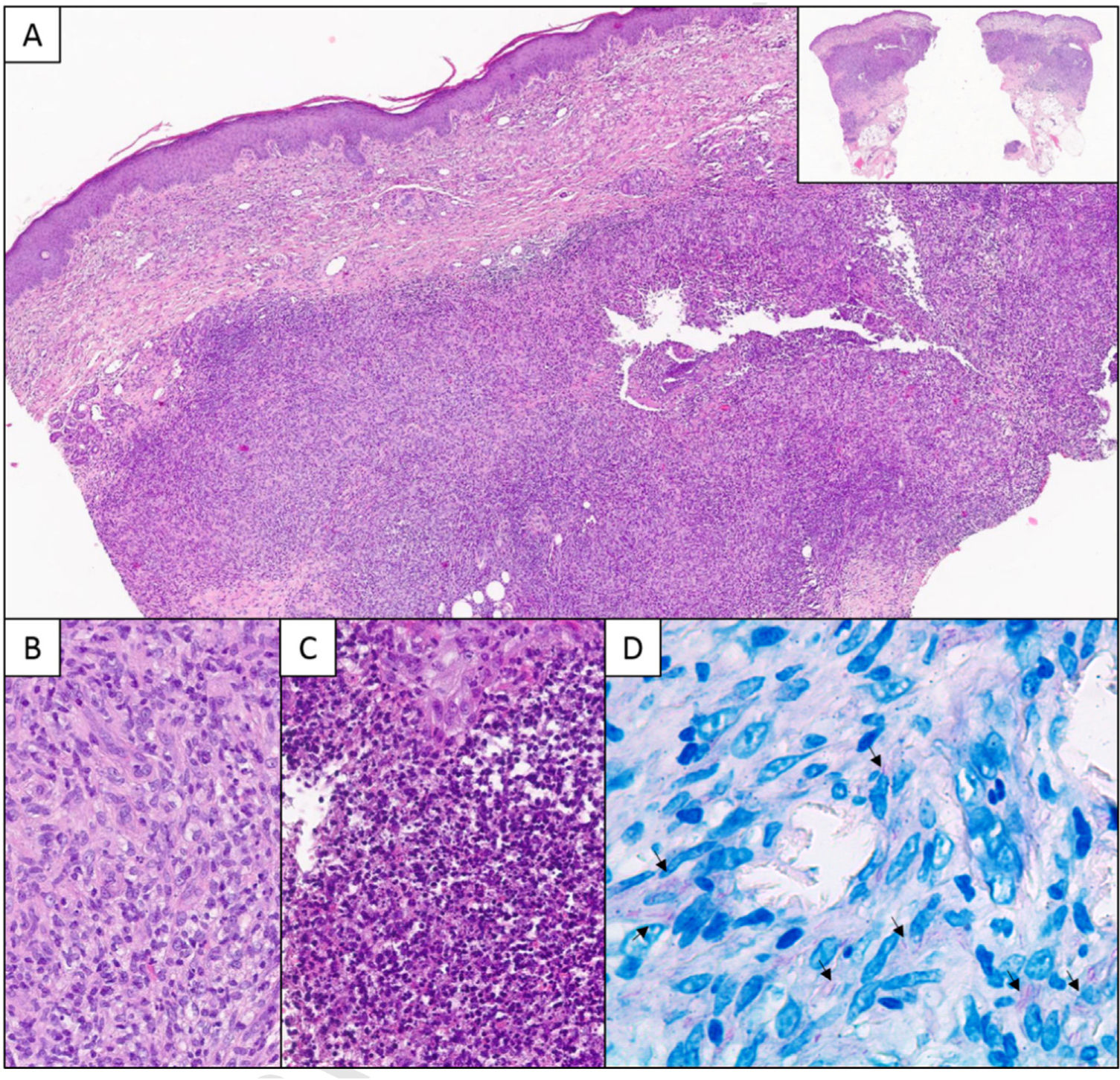
A, Histologic section showing a deep granulomatous dermal infiltrate with a nodular pattern (panoramic view in top-right corner) (hematoxylin–eosin, original magnification ×40). B, Detailed view showing a lymphocytic and histiocytic infiltrate (hematoxylin–eosin, original magnification ×200). C, Suppurative areas with abundant neutrophils and cell debris (hematoxylin–eosin, original magnification ×400). D, Ziehl–Neelsen staining. Note the long pink structures (arrows) (original magnification ×630).
The sporotrichoid pattern observed in M. chelonae infection is due to the ascending spread of the mycobacteria along the lymphatic channels.6 It is an unusual pattern, and just 15 cases have been reported in the literature (Table 1), none of them in a patient with sarcoidosis. The main entities to include in the differential diagnosis are infections due to other pathogens that present with a similar distribution, such as Sporothrix schenckii, Mycobacterium marinum, Nocardia species, and Leishmania species.
Cutaneous Mycobacterium chelonae Infections with a Sporotrichoid Distribution Reported in the Literature.
| Case | Age, y/sex | Location | Underlying disease | Immunosuppression | Treatment | Recurrence | Treatment after recurrence |
|---|---|---|---|---|---|---|---|
| Greer, 197912 | 76/F | Leg | No | Isoniazid+amithiozone | No | ||
| Higgins, 198813 | 65/F | Forearm | Chronic active hepatitis | Yes | Erythromycin+amikacin | No | |
| Murdoch, 198914 | 61/F | Leg | Kidney transplant | Yes | Pyrazinamide+rifampicin 6 mo | Yes | Erythromycin |
| Jopp-McKay, 199015 | 52/F | Leg | Kidney transplant | Yes | Minocycline 2 mo | Yes | TMP-SMX+surgery |
| Zahid, 199416 | 70/M | Hand | COPD | Yes | Ciprofloxacin+clarithromycin 6 mo | No | |
| Endzweig, 200117 | 59/M | Leg | Kidney transplant | Yes | Surgery+ciprofloxacin+TMP-SMX+imipenem | Yes | Surgery+amikacin+cefoxitin+clarithromycin |
| Haas, 200118 | 66/F | Forearm | Rheumatoid arthritis | Yes | TMP-SMX+clarithromycin | Yes | Azithromycin+ciprofloxacin+surgery |
| Demitsu, 200119 | 46/M | Forearms | Congestive heart failure Diabetes | No | Minocycline 2 mo | Yes | Surgery |
| Rosón, 200220 | 42/F | Forearm | No | Minocycline | No | ||
| Phillips, 200821 | 43/F | Forearm | Bilateral panuveitis | Yes | Imipenem+piperacillin-tazobactam+amoxicillin-clavulanic acid 5 mo | No | |
| de Vasconcelos, 201522 | 60/M | Forearm | Rheumatoid arthritis | Yes | Clarithromycin 6 mo | No | |
| Orrin, 201623 | 65/F | Leg | Cryptogenic organized pneumonia | Yes | Clarithromycin 9 mo | No | |
| Boulavsky, 201724 | 75/F | Leg and foot | Lupus nephritis | Yes | Clarithromycin+amikacin+levofloxacin | No | |
| Kemp, 20173 | 54/F | Forearm | Systemic lupus erythematosus | Yes | Linezolid+clarithromycin 4 mo | No | |
| DuBow, 20196 | 31/F | Leg | Systemic lupus erythematosus | Yes | Linezolid+clarithromycin 8 mo | Yes | Linezolid+clarithromycin 3 mo |
| Current case | 70/F | Forearm | SarcoidosisPrimary immunodeficiency | Yes | Clarithromycin 4 mo | Yes | Clarithromycin 2 mo+IVIG |
Abbreviations: COPD, chronic obstructive pulmonary disease; F, female; IVIG, intravenous immunoglobulin; M, male; TMP-SMX: trimethoprim-sulfamethoxazole.
Immune system alterations should be ruled out in patients with atypical mycobacterial infections, especially in the presence of an uncommon pattern, such as sporotrichoid spread. Skin infections are the most common dermatologic manifestations of PIDs. Susceptibility may be specific to certain pathogens, depending on which part of the immune system is compromised.7 Patients with PIDs caused by mutations in interferon γ genes, which are characterized by phagocyte defects without altered humoral immunity, are prone to severe disseminated infections caused by atypical mycobacteria.8 In our case, we observed IgG deficiency and B-cell lymphopenia. Antibody deficiencies are commonly associated with respiratory bacterial infections, which were also present in our patient's history.
Biopsy is key to diagnosis. Histopathologic patterns include a diffuse histiocytic infiltrate, microabscesses, panniculitis, tuberculoid or sarcoid granulomas, and/or reactive vasculopathy.9 Acid-fast bacilli are demonstrated by specific stains such as Ziehl-Neelsen, although a negative test does not rule out a mycobacterial infection.5,10 Diagnosis is confirmed by culture or molecular techniques such as PCR restriction fragment length polymorphism analysis.2,10
M. chelonae infections tend to have an unpredictable resistance profile, hence the importance of susceptibility testing. Although clarithromycin monotherapy is sufficient in most cases, combined therapy is recommended due to the risk of resistance developing during treatment, which is frequently administered for long periods.6,11 Adjuvant surgical treatment may be required in certain cases.5
In conclusion, when dealing with a patient with sporotrichoid cutaneous lesions, it is important to rule out an atypical mycobacteria infection, especially in immunosuppressed patients.