Sebaceous carcinoma (SC) is a rare but potentially aggressive malignant skin neoplasm, often categorized-based on its anatomical location-into ocular, or extraocular neoplasm. Both tumors behave differently, show as primary or secondary neoplasms, and be associated with the Muir–Torre syndrome (MTS).1
Patients diagnosed with SC registered in the pathology lab database of Hospital Universitario Fundación Alcorcón, Madrid, Spain from January 1998 through December 2022 were studied. Data were obtained after reviewing all health records, biopsies, and the patients’ demographic, clinical, histopathological characteristics, treatment, and evolution.
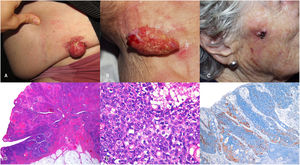
A total of 7 cases of SC diagnosed over the past 24 years were identified. The most relevant characteristics are seen in Table 1. The patients’ median age was 85 years (range from 55 to 94), 4 patients were men and 3 were women. In 6 of the cases, the tumor was found on the head and neck, while 2 were periocular tumors. Clinically, they showed as erythematous-orange papules or nodules, with a median size of 2cm (range from 0.4cm to 5cm) (Fig. 1A–C). The median time to diagnosis was 5 months (range from 3 to 36 months). Histopathological confirmation of neoplastic cells with sebaceous differentiation, with various degrees of differentiation and atypia, supported by immunohistochemical stains such as adipophilin, androgen receptors, or cytokeratin 5/6, was required for diagnosis (Fig. 1D–F).
Most relevant demographic characteristics.
| No. | Sex | Age, years | Location | Muir–Torrea | TAP | Treatmentc | Metastasis/relapse | Year |
|---|---|---|---|---|---|---|---|---|
| 1 | Man | 88 | Inner canthus | No | Normal | No | No | 2005 |
| 2 | Woman | 94 | Cheek | No | No | Yes | No | 2005 |
| 3 | Woman | 86 | Neck | No | Normal | Yes | No | 2008 |
| 4 | Man | 61 | Upper eyelid | Nob | No | Yes | No | 2015 |
| 5 | Man | 55 | Scalp | No | Normal | Yes | No | 2016 |
| 6 | Man | 85 | Scalp | Nob | Normal | Yes | No | 2020 |
| 7 | Woman | 85 | Abdomen | Nob | Normal | Yes | No | 2021 |
TAP, thoraco-abdominopelvic.
(A–C) Clinical images of 3 patients. (A) 5cm proliferative, exophytic, erythematous, friable, and ulcerated lesion in left hemiabdomen. (B) 4cm×2cm exophytic, pedunculated, erythematous, friable, and eroded lesion in right lateral cervical area. (C) 2cm erythematous, ulcerated, friable tumor on the right cheek. (D–F) Histopathological image. Hematoxylin and eosin stain. Panoramic view. Exophytic neoplastic proliferation with in situ component in left margin (D). Hematoxylin and eosin ×400. Areas of in situ sebaceous carcinoma built with mature and immature sebocytes (E). Immunohistochemical staining with adipophilin ×40. Immunoreactivity for adipophilin in mature sebocytes (F).
Two patients had a past medical history of cancer, without family history, 1 nephrectomized renal carcinoma, and 1 simultaneous diagnosis of melanoma, squamous cell carcinoma, and SC.
A thoraco-abdominopelvic CT scan was performed as an additional study in 5 out of the 7 patients, without any pathological findings being reported. Except for 1 patient who died before surgery due to sepsis, treatment was surgical, with wide excision until the fascial plane with clinical margins of 1cm. The patients’ median follow-up was 2 years (range from 6 months to 14 years), and no recurrences or metastases have been identified to this date in any of the patients. Two patients died within the first year after diagnosis due to infections (acquired pneumonia). None of the patients met the criteria for MTS according to the Mayo principles (Table 2).2 The microsatellite instability testing came out negative in all 3 patients.
Mayo Muir–Torre syndrome risk score.
| Variable | Score |
|---|---|
| Age at diagnosis (years)a | |
| ≥60 years 0 | 0 |
| <60 years 1 | 1 |
| Total no. of sebaceous neoplasms | |
| 1 | 0 |
| ≥2 | 2 |
| Personal history of a Lynch syndromeb-related cancer | |
| No | 0 |
| Yes | 1 |
| Family history of a Lynch syndrome-related cancer | |
| No | 0 |
| Yes | 1 |
Mayo Muir–Torre syndrome risk score. Scores of the 4 variables are added to create an overall score, with a possible range from 0 to 5. Scores≥2 have a sensitivity rate of 100% and a specificity rate of 81% to predict germline mutations in the Muir–Torre Syndrome.
SC has an estimated incidence rate of 2.4 cases per million individuals per year,3 and is often found in the periocular region in up to 75% of the cases reported.4 It is slightly more common in men (58%), with a mean age of 67.9 years.1
SC often starts as a painless, ulcerated nodule or papule of a red-orange color, and can disguise as various entities, such as blepharitis, chalazion, etc., which is why the definitive diagnosis is often delayed, thus increasing morbidity and mortality.1,4–6
Diagnosis requires histopathological confirmation of sebaceous differentiation. Well-demarcated tumors show microvacuolated clear cells with large, vesicular, or hyperchromatic nuclei with prominent nucleoli, which happen to be scarce or rare in the moderate or poorly differentiated ones. Due to the similarities to other skin neoplasms, immunohistochemistry can be very helpful (EMA [well-demarcated tumors], androgen receptors [nuclear staining], adipophilin [cytoplasmic vacuoles], and high molecular weight cytokeratins [CK 5/6]).1,4–6
Surgical excision is accepted as the gold standard for the management of SC, both ocular and extraocular, being Mohs micrographic surgery the first-line therapy.1,3–6 The 5-year overall survival rate of SC is 78% for localized and regional disease and 50% for metastatic disease, which is why close follow-up of patients is advised, with recommendations for check-ups every 6 months for 3 years and then on a yearly basis.1,3
MTS is a phenotypic variant of hereditary non-polyposis colorectal cancer (Lynch syndrome) due to dominantly inherited mutations in DNA repair genes and defined by an increased predisposition to sebaceous neoplasms.7–9 Although MSI (microsatellite instability) screening (MSH1, MSH2, MLH1, PMS2) was the previously advised screening method in all patients with SC,3 in the latest guidelines, SMT screening in periocular SC is ill-advised. Instead, individualized recommendations for extraocular SC are advised. Recent publications recommend initial screening with clinical criteria using the Mayo MTS risk score.2,10
In conclusion, the low incidence of SC and its nonspecific clinical presentation should tip us off on the need for establishing high clinical suspicion to avoid diagnostic and therapeutic delays, being histopathological examination being of paramount importance. We should mention that while routine screening for SMT is ill-advised, immunohistochemistry is a cost-effective additional test available in most laboratories in Spain and should be considered in all patients with sebaceous carcinomas. In full compliance with the latest clinical practice guidelines, we recommend initial screening using the Mayo MTS risk score, followed by immunohistochemistry, and genetic testing if criteria are met.
Conflicts of interestNone declared.