
Reticulated acanthoma with sebaceous differentiation (RASD) is a benign adnexal tumor first described by Ackerman et al. in 1998.1–3 Its incidence is unknown and it usually appears in the seventh decade of life, with a slight male predominance and a predilection for the trunk, head, and neck.3 Clinically, it presents as yellowish, brownish, or reddish papules or plaques.4,5 Dermoscopy usually shows a multicomponent pattern with bright yellowish anastomosing curvilinear structures or well-defined yellowish structures, with or without pigmented reticular lines, whitish reticular lines, and atypical vessels.4,5 Histologic findings are those of a benign epithelial tumor, with a proliferation of keratinocytes with a predominantly scaly appearance or more basaloid foci, interlinking the network of rete ridges associated with mature sebocytes.2–4
The association between sebaceous skin neoplasms and hereditary colorectal cancer, Muir Torre syndrome (MTS), is controversial, but these tumors are seen in approximately 5% of patients with Lynch syndrome (LS).6–8
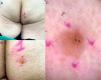
An 83-year-old woman presented with a gluteal RASD unrelated to MTS that was detected during routine follow-up for something else. The lesion was described as a scaly erythematous plaque with well-defined borders (Fig. 1A and B), and dermoscopy showed glomeruloid vessels. The tentative diagnosis was Bowen disease (Fig. 1C).
A and B, Slightly scaly erythematous yellowish plaque measuring 9mm×8mm in the right gluteal region. Note the well-defined borders and hyperpigmented area in the lower segment. C, Dermoscopy findings. Lesion with a pink background, some dotted and glomeruloid vessels, bright yellow anastomosing bundles, white and yellow dots, and blue-gray globules.
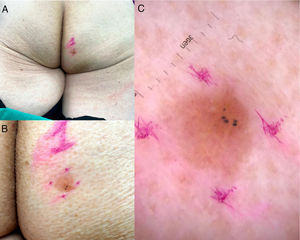
Histologic features were those of an adnexal epithelial tumor, comprising a proliferation of eosinophilic keratinocytes with several foci with a basaloid appearance (Fig. 2A) associated with clusters of sebocytes at the base of anastomosing epithelial cords (Fig. 2B and C). This latter finding was much more evident on immunohistochemical staining for androgen receptors (Fig. 2D). The papillary dermis contained several vessels with a reactive appearance, without atypia, and a sparse superficial perivascular mononuclear inflammatory infiltrate. No signs of malignancy were observed. Immunohistochemical studies for DNA mismatch repair proteins with intact nuclear expression were performed for MLH1, MSH2, MSH6, and PMS2 (Fig. 2E–H).
Reticulated acanthoma with sebaceous differentiation. A, Photomicrograph (hematoxylin–eosin [H–E], original magnification ×4). Neoplastic lesion with an adnexal epithelial appearance characterized by a proliferation of predominant eosinophilic keratinocytes. B, Photomicrograph (H–E, original magnification ×10). Small anastomosed foci with a more basaloid appearance. C, Photomicrograph (H–E, original magnification ×40). Clusters of sebocytes at the base of the anastomosing epithelial cords. D, Photomicrograph (original magnification ×10). Immunohistochemical staining for androgen receptors showing an evident sebaceous component. E, Photomicrograph (original magnification ×10). Immunohistochemical staining for MLH1 with an intact nuclear pattern. F, Photomicrograph (original magnification ×10). Immunohistochemical staining for MSH2 with an intact nuclear pattern. G, Photomicrograph (original magnification ×10). Immunohistochemical staining for MSH6 with an intact nuclear pattern. H, Photomicrograph (original magnification ×10). Immunohistochemical staining for PMS2 with an intact nuclear pattern.
RASDs are sporadic, slow-growing, and generally solitary benign tumors derived from the skin appendages.2,3,9
Diagnosis is usually delayed due to a low index of clinical suspicion.2 Clinically, the tumors resemble both benign and malignant skin lesions, and the main entities to consider in the differential diagnosis include seborrheic keratosis, sebaceous adenoma, Bowen disease, and basal cell carcinoma.2–4 Symptoms are linked to size, although RASDs are usually asymptomatic. Integration of clinical and dermoscopic findings can point to a diagnosis, but histologic confirmation is necessary.5,6
The most characteristic dermoscopic findings are well-defined yellowish areas, or anastomosing yellowish bundles that histologically correspond to the accumulation of sebocytes at the lower part of the tumor lobules. Reticular hyperpigmentation, corresponding to melanin-laden keratinocytes in histology, is also common.4,5
Histologically, RASD is seen as an epithelial lesion characterized by a proliferation of keratinocytes with elongation of the network of anastomosing epidermal rete ridges associated with sebaceous glands and mature sebaceous ductal structures in the basal layer.2–4 Poorly dilated capillary vessels and a mild mononuclear infiltrate were seen in the papillary dermis.2–4 Histologic differential diagnoses include seborrheic keratosis, which unlike RASD does not have a sebaceous component in the reticular cords; eccrine poroma, which shows a proliferation of poroid and cuticular cells and differs from RASD in that ducts are present and sebaceous differentiation is focal and uncommon; verruca vulgaris, which unlike RADS shows viral cytopathic changes and absence of a sebaceous component; and sebaceous nevus, which shows a distinctive papillomatous epithelial proliferation and has a larger sebaceous, eccrine, and apocrine component.
A diagnosis of RASD was established following integration of characteristic clinical, dermoscopic, and histopathologic findings.
The first published case of RASD was associated with MTS, which is characterized by the presence of sebaceous tumors and cancers associated with LS (e.g., cancers of the colon, endometrium, urothelium, and central nervous system).6–8 These diseases are caused by mutations in, or less commonly, hypermethylation of DNA mismatch repair genes (MLH1, MSH2, MSH6, and PMS2).6,7 The incidence of LS/MTS in patients with sebaceous tumors ranges from 14% to 50%; sebaceous adenoma is the most common tumor observed.6–8
Immunohistochemical studies can aid in the diagnosis of LS/MTS. They have a sensitivity of 81%–85% and a specificity of 48%6,7 and show loss of expression of at least 1 DNA repair protein.6,7,10 The Mayo MTS risk score has also been devised to help identify patients with sebaceous tumors at risk for MTS.7,8
It should be recalled that abnormal immunohistochemical findings are not diagnostic for LS and should be interpreted in association with family history and genetic tests.6,7,10
We have described a case of RASD not associated with MTS. We have also reviewed the diagnostic histopathologic characteristics of this relatively uncommon sebaceous neoplasm. RASD must be differentiated from epithelial tumors with a similar morphology, such as seborrheic keratosis, eccrine poroma, sebaceous nevus, and other sebaceous lesions or lesions with sebaceous differentiation. As RASD is associated with MTS, it is important to always take a complete clinical history, apply the Mayo MTS risk score, and perform immunohistochemical studies to check for risk factors for malignant tumors.
Conflicts of InterestThe authors declare that they have no conflicts of interest.

![Reticulated acanthoma with sebaceous differentiation. A, Photomicrograph (hematoxylin–eosin [H–E], original magnification ×4). Neoplastic lesion with an adnexal epithelial appearance characterized by a proliferation of predominant eosinophilic keratinocytes. B, Photomicrograph (H–E, original magnification ×10). Small anastomosed foci with a more basaloid appearance. C, Photomicrograph (H–E, original magnification ×40). Clusters of sebocytes at the base of the anastomosing epithelial cords. D, Photomicrograph (original magnification ×10). Immunohistochemical staining for androgen receptors showing an evident sebaceous component. E, Photomicrograph (original magnification ×10). Immunohistochemical staining for MLH1 with an intact nuclear pattern. F, Photomicrograph (original magnification ×10). Immunohistochemical staining for MSH2 with an intact nuclear pattern. G, Photomicrograph (original magnification ×10). Immunohistochemical staining for MSH6 with an intact nuclear pattern. H, Photomicrograph (original magnification ×10). Immunohistochemical staining for PMS2 with an intact nuclear pattern.](https://static.elsevier.es/multimedia/00017310/00000113000000S1/v1_202212200523/S0001731022008845/v1_202212200523/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



