Psoriasis is a multisystem disease associated with an increased prevalence of oral lesions. The aim of this study was to determine the prevalence of oral lesions in patients with psoriasis and examine associations with clinical and patient characteristics.
Material and methodsWe conducted a cross-sectional study of patients with psoriasis and healthy controls seen between December 2019 and February 2020. We recorded biometric data, comorbidities associated with psoriasis, oral examination findings, and clinical characteristics of psoriasis.
ResultsWe studied 100 patients with psoriasis and 100 controls. Oral lesions were more common in the psoriasis group (74% vs 46%, P<.001). The most common lesions were fissured tongue (39% vs 16%, P<.001) and periodontitis (28% vs 16%, P=.04). Geographic tongue was uncommon in both the study and the control group (4% vs 2%, P=.68). In the psoriasis group, patients with fissured tongue had a higher prevalence of cardiovascular disease (23.1% vs 4.9%), diabetes mellitus (28.2% vs 8.2%), and psoriatic arthritis (15.4% vs 1.6%) than those without this condition. Periodontitis was also associated with a higher prevalence of cardiovascular disease (28.6% vs 5.6%). Type of psoriasis, location, and time since onset were not significantly associated with oral lesions. Patients with oral lesions, however, had more severe disease (Psoriasis Area Severity Index [PASI], 3.9 vs 2.4; P=.05). Mean PASI was also higher in patients with fissured tongue (4.7 vs. 2.7, P=.03) and periodontitis (5.1 vs. 2.9, P=.04).
ConclusionsThe prevalence of oral lesions, especially fissured tongue and periodontitis, is higher in patients with psoriasis than in healthy controls. Oral lesions were associated with more severe psoriasis and a higher prevalence of associated comorbidities. We recommend examining the oral cavity of patients with psoriasis, especially those with more severe disease and comorbidities, irrespective of type of psoriasis, location, or time since onset.
La psoriasis es una enfermedad con una gran implicación sistémica en la que se ha descrito una mayor prevalencia de alteraciones de la mucosa oral. El objetivo del estudio fue determinar la prevalencia de alteraciones orales en pacientes con psoriasis y su asociación con las características clínicas y epidemiológicas de la enfermedad.
Material y métodosSe realizó un estudio transversal, incluyendo pacientes con psoriasis y voluntarios sanos entre diciembre de 2019 y febrero de 2020. Se recogieron datos biométricos, comorbilidades asociadas y alteraciones orales detectadas en la exploración física. Además, en los pacientes con psoriasis se registraron las características clínicas de la enfermedad.
ResultadosSe incluyeron 100 pacientes con psoriasis y 100 controles. Los pacientes con psoriasis presentaron una mayor prevalencia de alteraciones de la mucosa oral respecto al grupo control (74% frente a 46%, p<0,001), destacando una mayor prevalencia de lengua fisurada (LF) (39% frente a 16%, p<0,001) y periodontitis (28% frente a 16%, p=0,04). La lengua geográfica resultó poco frecuente en ambos grupos (4% frente a 2%, p=0,68). Dentro del grupo con psoriasis, los pacientes con LF presentaron mayor prevalencia de enfermedad cardiovascular (23,1% frente a 4,9%), diabetes mellitus (28,2% frente a 8,2%) y artritis psoriásica (15,4% frente a 1,6%) que aquellos sin LF. Los pacientes con periodontitis presentaron también mayor frecuencia de enfermedad cardiovascular (28,6% frente a 5,6%). La forma, la localización y el tiempo de evolución de la psoriasis no se relacionaron con más alteraciones orales. Sin embargo, los pacientes con alteraciones orales presentaron valores medios de la escala PASI (índice de severidad del área de psoriasis) más elevados (3,9 frente a 2,4, p=0,05). Los pacientes con LF presentaron un PASI más elevado que aquellos sin LF (4,7 frente a 2,7, p=0,03). Asimismo, los pacientes con periodontitis presentaron mayor gravedad de la enfermedad en comparación con aquellos sin esta manifestación (5,1 frente a 2,9, p=0,04).
ConclusionesLos pacientes con psoriasis tienen una mayor prevalencia de alteraciones orales, especialmente LF y periodontitis. Estos pacientes también presentaron una psoriasis más grave y más comorbilidades asociadas. Consideramos conveniente realizar una exploración oral a los pacientes con psoriasis, especialmente en aquellos de mayor gravedad y comorbilidades asociadas, independientemente del tiempo de evolución, forma o localización de la enfermedad.
Psoriasis is an immune-mediated chronic inflammatory skin disease with a prevalence between 0.9% and 8.5% of the adult population worldwide.1 It has traditionally been considered a skin disease. Today, however, it is considered to be a systemic disease, as these patients present a high prevalence of associated comorbidities, such as psoriatic arthritis, metabolic syndrome, cardiovascular disease, nonalcoholic fatty liver disease, inflammatory bowel disease, osteoporosis, psychiatric diseases, smoking, and alcohol abuse.2–6
Moreover, a higher, highly variable, prevalence of abnormal oral mucosa has been reported in patients with this disease. In the literature, fissured tongue (FT) is the most frequently reported oral abnormality associated with psoriasis, followed by geographic tongue (GT).7 More recently, a higher prevalence of periodontitis has been identified in these patients.8,9 Other oral manifestations, whose association is inconclusive, have been studied, including cheilitis, leukokeratosis, erythematous macules, fibromas, and depapillation of the tongue.
The existence of oral psoriasis lesions is a subject of debate. These lesions have been described in the literature as oral psoriasis because they present histologic findings similar to psoriasis and have a parallel clinical course.10 The patterns of the lesions vary from raised, white, desquamative lesions on the palate or buccal mucosa, to well defined, flat, erythematous lesions that affect the dorsal surface of the tongue with an annular or serpiginous edge.11 Bruce et al.12 considered that the low frequency of oral psoriasis may be explained by its transitory course, given the high rate of epidermal replacement in psoriasis and the asymptomatic and nonspecific nature of the lesions.
Oral abnormalities in this group of patients appear to be frequent and underdiagnosed, although little information in this regard is available in the literature.13
The objective of our study is to determine the prevalence of oral abnormalities in patients with psoriasis and their association with the clinical and epidemiologic characteristics of the disease.
Materials and MethodsWe performed a cross-sectional study of a group of patients with a clinical diagnosis of psoriasis and a control group. Patients who attended hospital outpatient consultations in the dermatology department of Hospital Universitari Sagrat Cor, Barcelona, Spain between December 2019 and February 2020 were enrolled in the study.
Inclusion criteria were age over 18 years and, in the psoriasis group, diagnosis by a dermatologist. Exclusion criteria were pregnant patients, other associated inflammatory skin disease, and prior disease of the oral mucosa. The control group consisted of patients with no personal or family history of psoriasis, who visited our department for reasons not related to inflammatory disease. A control patient was enrolled for every patient with psoriasis.
The principal variable was the presence of abnormal oral mucosa observable at the time of the consultation. Secondary variables were age, gender, and comorbidities. Moreover, the following clinical characteristics of the disease were recorded in the group of patients with psoriasis: time since onset, form of involvement, special locations, severity assessed using the PASI (psoriasis area severity index), BSA (body surface area), and DLQI (dermatology life quality index) scores, and prior treatments and current treatment.
The data were collected using a questionnaire completed by the physician during the consultation based on the patient's history, the physical cutaneous examination, and examination of the oral mucosa.
A statistical analysis was performed expressing the qualitative variables as number and percentage, and the quantitative variables as mean and standard deviation. In the univariate analysis, the χ2 test was used for qualitative variables, and the t test was used for quantitative variables, and the corresponding tests were used if the conditions of application were not met. The magnitude of the effect was expressed as the odds ratio (OR), with the corresponding 95% confidence interval (95% CI); logistic regression models were used to adjust the effect in the multivariate analysis, using the likelihood ratio and the principle of parsimony as the criteria for goodness of fit. A value of P<.05 was considered to be statistically significant. Statistical analysis of the data was performed using the IBM SPSS statistical software package (version 23, IBM Corp., NY, United States of America).
The study was approved by the Grupo Hospitalario Quirónsalud-Catalunya research ethics committee (GMN-INF-01-2019), in compliance with the Declaration of Helsinki and the legislation in force. All patients provided written informed consent in order to take part.
ResultsA total of 100 patients were enrolled in the psoriasis group and 100 patients were enrolled in the control group. The epidemiologic characteristics and disease history of the patients are shown in Table 1.
Epidemiologic Characteristics and History of the Patients.
| Psoriasis | Control | P | |
|---|---|---|---|
| n=100 | n=100 | ||
| Sex | |||
| Male (%) | 54 (54) | 48 (48) | |
| Female (%) | 46 (46) | 52 (52) | .4 |
| Age, y | |||
| Mean (SD) | 54.7 (16.3) | 64.7 (18.4) | <.01 |
| History | |||
| Smoker (%) | 30 (30) | 17 (17) | .03 |
| Ex-smoker (%) | 34 (34) | 34 (34) | 1 |
| Alcoholism (%) | 17 (17) | 15 (15) | .7 |
| Diabetes mellitus (%) | 16 (16) | 6 (6) | .02 |
| Hypertension (%) | 39 (39) | 39 (39) | 1 |
| Dyslipidemia (%) | 36 (36) | 45 (45) | .19 |
| Obesity (%) | 35 (35) | 16 (16) | <.01 |
| Cardiovascular disease (%) | 12 (12) | 19 (19) | .17 |
| Nonalcoholic fatty liver disease (%) | 11 (11) | 3 (3) | .05 |
| Chronic renal failure (%) | 2 (2) | 2 (2) | 1 |
| Recurrent aphthous stomatitis (%) | 1 (1) | 0 (0) | 1 |
| Psychiatric disorder (%) | 22 (22) | 12 (12) | .06 |
| Psoriatic arthritis (%) | 7 (7) | 0 (0) | .01 |
| Osteoporosis (%) | 4 (4) | 5 (5) | 1 |
| Inflammatory bowel disease (%) | 0 (0) | 2 (2) | .5 |
Data are expressed as absolute values and frequencies (%). The Pearson χ2 test and the Fisher exact test were used, as appropriate, for qualitative variables and the t test was used for quantitative variables.
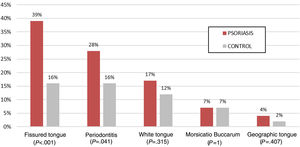
Table 2 shows the prevalence of oral manifestations in the patients with psoriasis and in the control group. The patients with psoriasis presented a greater prevalence of abnormal oral mucosa (74%) compared to the control group (46%), with a raw OR of 3.3 (95% CI, 1.8–6.1; P<.001). The magnitude of the effect was maintained after adjusting for age, sex, smoking, diabetes mellitus, obesity, and nonalcoholic fatty liver disease (OR, 3.1; 95% CI, 1.6–6.1; P=.001). The patients with psoriasis also had a greater prevalence of FT (39% compared to 16%; OR, 3.4; 95% CI, 1.7–6.6; P<.001), which was maintained in the logistic regression after adjusting for the same variables as in the previous case (OR 3.1; 95% CI, 1.4–6.9; P=.004), and of periodontitis (28% versus 16%; OR, 2.0; 95% CI, 1.0–4.1; P=.041), although statistical significance was lost after adjusting for the same variables (OR, 2.1; 95% CI, 0.9–4.8; P=.076) (Fig. 1). The between-group differences in the other oral mucosa abnormalities were not statistically significant (Table 2).
Prevalence of Oral Manifestations.
| Psoriasis | Control | OR (95% CI) | P | |
|---|---|---|---|---|
| n=100 | n=100 | |||
| Presence of ≥1 oral lesions* | 74 (74%) | 46 (46%) | 3.3 (1.8–6.1) | <.001 |
| Fissured tongue | 39 (39%) | 16 (16%) | 3.4 (1.7–6.6) | <.001 |
| Periodontitis | 28 (28%) | 16 (16%) | 2.0 (1.0–4.1) | .041 |
| White tongue | 17 (17%) | 12 (12%) | 1.5 (0.8–3.3) | .315 |
| Morsicatio buccarum | 7 (7%) | 7 (7%) | 1 (0.3–2.9) | 1 |
| Geographic tongue | 4 (4%) | 2 (2%) | 2.0 (0.4–11.4) | .407 |
| Angular cheilitis | 3 (3%) | 0 (0%) | 1.0 (0.9–1.1) | .081 |
| Papillary hypertrophy | 3 (3%) | 0 (0%) | 1.0 (0.9–1.1) | .081 |
| Black hairy tongue | 2 (2%) | 0 (0%) | 1.0 (0.9–1.0) | .155 |
| Cheilitis | 2 (2%) | 0 (0%) | 1.0 (0.9–1.1) | .155 |
| Fordyce spots | 2 (2%) | 0 (0%) | 1.0 (0.9–1.1) | .155 |
| Fibroma | 2 (2%) | 1 (1%) | 2 (0.2–22.7) | .561 |
| Median rhomboid glossitis | 1 (1%) | 0 (0%) | 2 (0.9–1.0) | .316 |
| Canker sores | 0 (0%) | 1 (1%) | 0.9 (0.9–1.0) | .316 |
| Herpes | 0 (0%) | 1 (1%) | 0.9 (0.9–1.0) | .316 |
| Venous lake | 0 (0%) | 1 (1%) | 0.9 (0.9–1.0) | .316 |
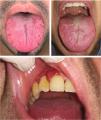
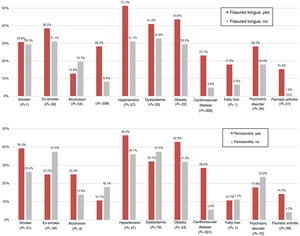
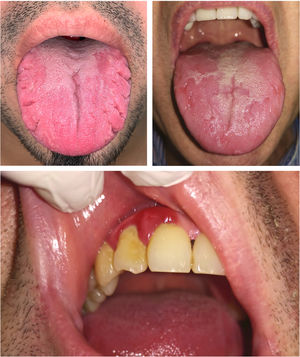
In the psoriasis group, patients with FT (Fig. 2) presented a greater frequency of cardiovascular disease (23.1% vs. 4.9%; OR, 5.8; 95% CI, 1.5–23.0; P=.006), diabetes mellitus (28.2% vs. 8.2%; OR, 4.4; 95% CI, 1.4–13.9; P=.008), and psoriatic arthritis (P=.01) in comparison with patients without FT. Furthermore, patients with periodontitis presented a greater prevalence of cardiovascular disease than patients without periodontitis (28.6% vs. 5.6%; OR, 6.8; 95% CI, 1.8–24.9; P=.001) (Fig. 2). When the effect was adjusted using logistic regression, patients with FT continued to present significantly more cardiovascular disease after adjusting for age, sex, smoking, and diabetes mellitus (OR, 11.3; 95% CI, 1.7–73.1; P=.011), and significance was lost in the case of diabetes after adjusting for age, sex, and smoking (OR, 0.9; 95% CI, 0.2–5.3; P=.963). In the case of periodontitis, statistical significance was also maintained after adjusting for age, sex, smoking, and diabetes mellitus (OR, 10.5; 95% CI, 1.9–55.7; P=.006) (Fig. 3).
The form, location, and time since onset of the psoriasis were not associated with a higher frequency of oral abnormalities, although patients with periodontitis appear to have a higher frequency of palmoplantar psoriasis than those without periodontitis (P=.05) (Table 3). We observed no link between oral abnormalities and patients’ prior or current treatments. Patients with oral abnormalities presented greater severity of disease in comparison with patients without oral manifestations, as reflected in higher PASI and BSA scores. Moreover, patients with FT presented a higher PASI score than those without FT (4.7 vs. 2.7; P=.03). Patients with periodontitis also presented a higher PASI score than those without periodontitis (5.1 vs. 2.9; P=.04) (Table 4).
Prevalence of Most Frequent Oral Manifestations by Form of Psoriasis, Special Locations, and Time Since Onset.
| Oral manifestationsn (%) | Fissured tonguen (%) | Periodontitisn (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | |
| Form of psoriasis | |||||||||
| Plaque (n=81) | 60 (74.1) | 21 (25.9) | .97 | 33 (40.7) | 48 (59.3) | .46 | 23 (28.4) | 58 (71.6) | .86 |
| Guttate (n=22) | 16 (72.7) | 6 (27.3) | .88 | 5 (22.7) | 17 (77.3) | .08 | 6 (27.3) | 16 (72.7) | .93 |
| Flexural (n=10) | 7 (70) | 3 (30) | .70 | 6 (60) | 4 (40) | .15 | 2 (20) | 8 (80) | .72 |
| Palmoplantar (n=6) | 6 (100) | 0 (0) | .34 | 3 (50) | 3 (50) | .67 | 4 (66.7) | 2 (33.3) | .05 |
| Erythroderma (n=3) | 3 (100) | 0 (0) | .57 | 2 (66.7) | 1 (33.3) | .56 | 2 (66.7) | 1 (33.3) | .19 |
| Pustular (n=2) | 2 (100) | 0 (0) | 1 | 2 (100) | 0 (0) | .15 | 0 (0) | 0 (0) | 1 |
| Special locations | |||||||||
| Scalp (n=76) | 57 (75) | 19 (25) | .69 | 31 (40.8) | 45 (59.2) | .63 | 22 (28.9) | 54 (71.1) | .71 |
| Nails (n=40) | 33 (82.5) | 7 (17.5) | .11 | 19 (47.5) | 21 (52.5) | .16 | 14 (35) | 26 (65) | .2 |
| Face (n=32) | 27 (84.3) | 5 (15.6) | .11 | 13 (40.6) | 19 (59.4) | .89 | 10 (31.2) | 22 (68.8) | .62 |
| Genitals (n=18) | 13 (72.2) | 5 (27.8) | .85 | 5 (27.8) | 13 (72.2) | .28 | 4 (22.2) | 14 (77.8) | .77 |
| Time since onset | |||||||||
| <1 year (n=5) | 3 (60) | P*=.32 | 0 (0) | P*=.1 | 0 (0) | P=.38 | |||
| 1–5 years (n=9) | 6 (66.7) | 2 (22.2) | 3 (33.3) | ||||||
| 5–10 years (n=14) | 11 (78.6) | 6 (42.9) | 5 (35.7) | ||||||
| 10–20 years (n=24) | 16 (66.7) | 11 (45.9) | 4 (16.7) | ||||||
| >20 years (n=48) | 38 (79.2) | 20 (41.7) | 16 (33.3) | ||||||
Data are expressed as absolute values and frequencies (%). The Pearson χ2 test and the Fisher exact test were used, as appropriate.
Psoriasis Severity by Most Frequent Oral Abnormalities in These Patients.
| Oral manifestations | Fissured tongue | Periodontitis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | |
| PASI (mean) | 3.9 | 2.4 | .051 | 4.7 | 2.7 | .031 | 5.1 | 2.9 | .042 |
| BSA (mean) | 5.3 | 3.1 | .041 | 5.6 | 4.2 | .254 | 6.9 | 3.9 | .082 |
| DLQI (mean) | 3.7 | 2.8 | .503 | 4.6 | 2.8 | .156 | 4.2 | 3.2 | .451 |
| n=74 | n=39 | n=28 | |||||||
Abbreviations: BSA indicates body surface area; DLQI, dermatology life quality index; PASI, psoriasis area severity index.
Values are expressed as mean. The t test was used for independent samples.
Our study shows that patients with psoriasis present a high prevalence of oral abnormalities in comparison with the healthy population, highlighting the presence of FT and periodontitis.
Previous studies that investigate oral abnormalities in patients with psoriasis are observational and have a small number of patients.13,14 Those studies also show heterogeneous results and mainly focus on the tongue disease. Moreover, some of them lack a control group and few assess the link between the abnormalities found and the characteristics of the psoriasis.
A strength of our study is the assessment of the entire oral disease and comparison with a control group without psoriasis. Furthermore, all assessments were performed by dermatologists, facilitating the inclusion of clinical data on the disease, patient quality of life, and severity of the psoriasis using standardized scales.
In terms of tongue abnormalities, FT is the most frequent abnormality in patients with psoriasis. Studies in different populations have found a prevalence of between 14% and 47% in these patients,7,13–16 similar to that observed in our study.
GT is the most widely studied oral abnormality in psoriasis, and its prevalence has been calculated to be between 5% and 18% in patients with psoriasis.17 Its association has been justified based on its fundamental lesions, which show similar microscopic alterations, and due to the presence of a common genetic marker, HLA-Cw6.18,19 Chewing and speaking are factors that produce microtrauma to the tongue, which may correspond to the Koebner phenomenon, stimulating the appearance of GT.
GT is frequently transitory, whereas FT tends to be permanent. This may explain the findings of Picciani et al.,20,21 who linked GT with psoriasis in the initial stages and FT with psoriasis a long time after onset, findings that we did not observe. This temporary course of GT may also explain the low prevalence in our study, as almost half the patients had psoriasis that had appeared more than 20 years earlier.
In the study by Tomb et al.,13 patients with pustular psoriasis presented a higher prevalence of FT, results similar to those obtained by Daneshpazhooh et al.15 Although all the patients with pustular psoriasis in our study present FT, the small number of patients enrolled with this form of psoriasis does not allow us to reach conclusions.
In the literature, the presence of GT has been linked to the severity of psoriasis. Given that severe forms of the disease present twice the prevalence of mild forms, some authors consider it to be a marker of severity.19 The frequency of GT has been observed to be higher in patients with a PASI score of greater than 10 and a DLQI score greater than 10.15,22 In our study, patients with FT presented more severe disease than those without FT and patients with periodontitis also presented more severe disease than those without periodontitis. As in the literature, the presence of oral manifestations was associated with more severe psoriasis.15,21 These findings may be explained by shared pathophysiological mechanisms and suggest that oral involvement may be a marker of severity in psoriasis.
We found no significant differences between patients with involvement of the scalp, nails, face, or genitals. Keshavarz et al.,23 however, found more GT and FT in patients with facial involvement, and Zargari24 in patients with nail or genital involvement.
Moreover, an increase in periodontal disease has recently been observed with psoriasis.25 Similarly, in our study, periodontitis was the second most frequent abnormality in these patients. Egeberg et al.26 also observed a higher risk of periodontitis in patients with psoriasis and psoriatic arthritis, which increased with the severity of the disease. Ganzetti et al.9 observed a higher concentration of proinflammatory cytokines, especially IL-1 and tumor necrosis factor-ɑ in the saliva of patients with psoriasis. Histologically, both entities involve neutrophils,27 and recent studies have shown an important role of IL-17 in the development of periodontitis,28 as also occurs in psoriasis.29 Furthermore, Dalmády et al.30 suggest that the presence of periodontitis may exacerbate the psoriasis by activation of the Th17/IL-17 immune pathway. This histologic and pathogenic similarity between the 2 diseases may explain their direct relationship. Thus, in agreement with Woeste et al.,31 we consider it necessary to include periodic dental check-ups in patients with psoriasis, especially those with more severe disease. Early detection and treatment may lead to an improvement in the quality of life of these patients.
Moreover, in our study, we observed that patients with FT and periodontitis present comorbidities more often than those without these oral manifestations.
Limitations of our study include the small sample size, the interpersonal variability of the assessments, and the cross-sectional design.
In conclusion, patients with psoriasis have a greater prevalence of oral abnormalities, especially FT and periodontitis. These patients also present more severe psoriasis and more associated comorbidities. We therefore consider an oral examination to be necessary in all patients with psoriasis. The oral examination should be performed regardless of time since onset, form, or location of the psoriasis. Nevertheless, studies with a larger sample size and follow-up over time are required.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors would like to thank Dr. José Miguel Baena Díez for his help in performing the statistical analysis.