Vitiligo is a pigmentary disorder, in which oxidative stress has been evidenced as part of the pathogenesis. Pathways responsible for protecting melanocytes from damage caused by reactive oxygen species are known as the nuclear factor erythroid factor 2 (Nrf2) pathway.
Nrf2 is a transcription factor that remains inhibited when the organism is in homeostasis, but in the presence of oxidative stress it allows the encoding of phase ii antioxidant enzymes.
In vitiligo there are abnormalities in the location and function of Nrf2 as well as polymorphisms that increase the risk of this disease. Currently, multiple molecules that act on Nrf2 have been investigated in order to find useful emerging treatments for vitiligo.
A search for articles in Spanish and English was carried out in the PubMed, Ovid, Scopus and Web of Science Clarivate databases, using the keywords “Vitiligo AND nuclear factor erythroid derived 2 like 2 OR NRF2” without time restriction. All in vitro studies, narrative reviews, case series, cohort studies, and randomized and non-randomized clinical trials that specifically addressed the issue of Nrf2 associated with vitiligo were included.
El vitíligo es un trastorno pigmentario en el que se ha evidenciado el estrés oxidativo como parte de la patogenia. Se conocen vías encargadas de proteger a los melanocitos del daño causado por las especies reactivas de oxígeno, como por ejemplo la vía del factor nuclear eritroide similar al factor 2 (Nrf2).
El Nrf2 es un factor de transcripción que cuando el organismo se encuentra en homeostasis permanece inhibido, pero en presencia de estrés oxidativo permite la codificación de enzimas antioxidantes de fase II.
En el vitíligo se evidencian anomalías en la localización y función del Nrf2, así como polimorfismos que aumentan el riesgo de esta enfermedad. Así mismo, se han investigado múltiples moléculas que actúan en el Nrf2 buscando encontrar tratamientos emergentes útiles para el vitíligo.
Se realizó una búsqueda de artículos en español e inglés en las bases de datos PubMed, Ovid, Scopus y Web of Science Clarivate, utilizando las palabras clave «Vitiligo AND nuclear factor erythroid derived 2 like 2 OR NRF2» sin restricción de tiempo. Se incluyeron todos los estudios in vitro, revisiones narrativas, series de casos, estudios de cohorte y ensayos clínicos aleatorizados y no aleatorizados que abordaban específicamente el tema del Nrf2 asociado a vitíligo.
Vitiligo is a multifactorial skin disorder, characterized by white spots of variable appearance and course, caused by loss of melanocyte function. It affects approximately 1% of the world population. The exact etiology of the condition is not fully known, but there is evidence that oxidative stress triggers the lesion in pigment-producing cells of the skin, thereby compromising their survival and function. Reactive oxygen species (ROS) can impact melanocyte metabolism, proliferation, and differentiation, leading to an immune response and generation of a proinflammatory environment conducive to tissue damage.1,2
The capacity of melanocytes to adapt to their environment and the presence of antioxidant and oxidant molecules to maintain the equilibrium in the medium are essential requirements for cell survival. There are pathways dedicated to protecting melanocytes from cell damage, such as nuclear factor erythroid 2-related factor 2 (Nrf2), which is a potent cell redox regulator.3
Pathogenesis of vitiligoFour theories have been proposed to explain the pathogenesis of vitiligo based on genetics, immunology, oxidative stress, and generation of inflammatory mediators. None of these theories alone is sufficient to explain the origin of vitiligo, although currently its autoimmune nature and multifactorial etiology are recognized.4,5 Twenty percent of patients with vitiligo have at least one first-degree relative also affected. A relative risk of presenting vitiligo in first-degree relatives is 7 to 10 times greater than in the overall population. Identical twins have a concordance rate of 23%, indicating that environmental factors are essential for the condition to develop. Around 50 different genetic loci have been associated with innate and adaptive immune regulation, melanogenesis, and cell apoptosis, and these are risk factors for developing vitiligo. An example is the TYR gene, which encodes a tyrosine that acts as an autoantigen in these patients. Another example is the NALP1 gene, which encodes the NACHT leucine-rich-repeat protein 1.4 This protein is a regulator of innate immune response and has also been associated with the development of vitiligo and other diseases and autoimmune syndromes.
Vitiligo and oxidative stressOxidative stress has been proposed as a possible trigger event for melanocyte destruction. The antioxidant system in melanocytes of patients with vitiligo is out of equilibrium, with high levels of markers of oxidative stress and significant depletion of antioxidant mechanisms6; this situation has been associated with a greater intrinsic sensitivity of melanocytes to external pro-oxidative stimuli. In addition, at the edges of the spots and in uninvolved skin of patients with vitiligo, a decreased adhesion between melanocytes and keratinocytes has been detected. This has been associated with the Koeber phenomenon. The mitochondria of patients with vitiligo appear to play a key role in oxidative stress, as they have abnormalities in transmembrane potential and in calcium channels in the cell membrane, in turn triggering cell apoptosis and oxidation. It has also been reported that the endoplasmic reticulum of melanocytes in these patients is dilated and houses certain molecules, such as calreticulin, which is expressed in states of oxidative stress. Overproduction of ROS stimulates the melanocytes to secrete exosomes with melanocyte-specific antigens. These antigens are presented to the dendritic cells, which activate T helper cells and cause regulatory T-cell dysfunction. The CD8 T cells in vitiligo lesions produce cytokines such as IFN-γ, which activate pathways such as JAK-STAT and ultimately lead to secretion of multiple proinflammatory cytokines.4
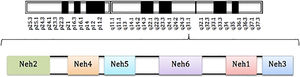
Definition and structure of nuclear factor erythroid 2-related factor 2Nrf2 is a transcription factor belonging to the cap’n’collar family. The gene that encodes this transcription factor is located on chromosome 2q31 and consists of 5 exons and 4 introns.1,7
The Nrf2 transcription factor is formed of 5 domains denominated Neh and a leucine zip domain (bZIP). These domains have specific functions and also include motifs of amino acids and lysine residues. Of the named domains, Neh2 is considered the principle one, as it is essential for activation or inhibition of this transcription factor (Fig. 1).3
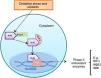
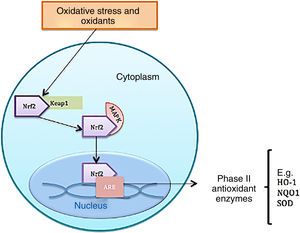
Function of nuclear factor erythroid 2-related factor 2Under homeostatic conditions, Nrf2 activity is constantly inhibited by binding to a cytoplasmic protein known as Kelch-like ECH-associated protein 1 (KEAP1), which is an adaptive subunit of E3 ubiquitin ligase whose function is to add chains of ubiquitin to Nrf2 as a marker for degradation by the proteosome. The half-life of Nrf2 in normal conditions is 20min; however, under oxidative stress, oxidants bind to Keap1 cysteine sensors triggering a confirmational change to allow Nrf2 to dissociate from the inhibitory protein; Nrf2 is consequently stabilized and translocates to the nucleus.8 Once in the cell nucleus, it forms a complex by heterodimerization with musculoaponeurotic fibrosarcoma proteins. These heterodimers recognize antioxidant response elements (ARE) located in the DNA promotor region of the genes, which encode phase ii antioxidant enzymes, such as heme oxygenase 1, superoxide dismutase, quinone oxidoreductase 1, NADH, the catalytic subunit of cysteine ligase glutamate, and the modulator subunit of glutamyl cysteine ligase, as well as nonenzymatic antioxidant proteins, such as thioredoxin or ferritin; these molecules act by neutralizing ROS through elimination and reduction reactions (Fig. 2 and Table 1).8–10
Description of studies contributing to knowledge of Nrf2 function.
| Author/year | Country | LE/SR | Type of study | Contribution |
|---|---|---|---|---|
| Königsberg Fainstein,3 2007 | Mexico | 5/D | Narrative review | Mechanism of Nrf2 regulation and activation |
| Schallreuter et al.,6 1991 | Germany | NA | Molecular biology study | Oxidants in patients with vitiligo |
| Song et al.,7 2016 | China | 3b/B | Case-control | Polymorphism in the Nrf2 gene that impacts susceptibility to vitiligo |
| Gęgotek and Skrzydlewska,9 2015 | Poland | 5/D | Narrative review | Action of Nrf2 on cell metabolism |
| Mou et al.,13 2018 | China | NA | Molecular biology study | Decreased Nrf2 expression due to increased HMGB1 |
| Jian et al.,14 2014 | China | 4/C | Longitudinal | Nrf2 cell expression and translocation in response to H2O2 |
| He et al.,15 2017 | China | NA | Molecular biology study | Deregulation of the Nrf2-p62 pathway in vitiligo melanocytes |
| Kim et al.,16 2014 | Korea | NA | Molecular biology study | PAR-2 molecule and its action on increasing Nrf2 nuclear translocation |
| Guan et al.,17 2008 | China | 3b/B | Case-control | Polymorphism in the Nrf2 gene that impacts susceptibility to vitiligo |
Abbreviations: HMGB1, high-mobility group B1 protein; H2O2, hydrogen peroxide; LE/SR, level of evidence and strength of recommendation based on the Oxford Centre for Evidence-Based Medicine scale; NA, not applicable, it meets one of the 11 Bradford Hill causality criteria (biological plausibility); Nrf2, nuclear factor erythroid 2-related factor 2; PAR-2, protease activated receptor 2.
Some other proteins, such as those that participate in the mitogen-activated kinase pathways, for example ERK, JNK, p62, and phosphatidylinositol 3-kinase, are important regulators of Nrf2 prior to its activation or degradation as they participate in the dissociation of Nrf2 from Keap1 and its subsequent nuclear translocation.8,11
Other additional mechanisms of action for Nrf2 activation are histone acetylation/deacetylation, Bach1 phosphorylation, and participation of the cell autophagy marker.12,13
Nuclear factor erythroid 2-related factor 2 and vitiligoNrf2 transcription factor is localized mainly in the cytoplasm of melanocytes in patients. In contrast, in healthy controls, the greater proportion of Nrf2 is located in the nucleus; in addition, Nrf2 levels in healthy controls exposed to hydrogen peroxide are higher compared with patients. This suggests that nuclear translocation of Nrf2 in response to oxidative stress is relatively reduced in patients with vitiligo, leading to a disequilibrium in the redox system.14 Therefore, methods have been investigated to increase Nrf2 activation. Recent studies have identified glycogen kinase 3 beta synthase as a new regulator of Nrf2. This agent acts by promoting accumulation of the transcription factor in the nucleus through phosphorylation.13 Another study conducted in Asian patients demonstrated that inhibition of the high mobility group 1 protein, a molecule released by immune system cells in the face of oxidative stress and that in normal conditions acts as a proinflammatory mediator, significantly increases expression of Nrf2.13
In addition to the classic antioxidant system, the cell autophagy mechanism can protect melanocytes from oxidative stress. This mechanism consists of grouping the intracellular organelles and proteins in autophagosomes to be degraded; it is controlled by the Nrf2-p62 pathway and is downregulated in patients with vitiligo.15
Altered Nrf2 function has not only been observed in pigment producing cells but also in keratinocytes of patients with vitiligo, where an increase in cell apoptosis and impaired cell viability have been observed. Kim et al.16 therefore investigated stimulation of protease activated receptor 2 in keratinocytes of 8 patients with stable nonsegmental vitiligo. The authors reported that this receptor can be activated and stabilize Nrf2 through accelerated degradation of Keap1, thereby allowing subsequent induction of phase ii enzymes; moreover, the study found that expression of protease-activated receptor 2 and Nrf2 was lower in lesioned skin compared with lesion-free skin in the same patient with vitiligo.
In addition to abnormalities in transcription factor localization and function, polymorphisms have been detected in the Nrf2 gene promoter region, such as T to C substitution at position 653, G to T substitution at position 617, and C to A substitution at position 650. These polymorphisms increase the risk of developing vitiligio.7,17
Molecules that act on nuclear factor erythroid 2-related factor 2 in patients with vitiligoPhenols such as monobenzyl ether of hydroquinone and 4-tertiaryl butylphenol are cytotoxic molecules that were found to increase nuclear translocation and activation of Nrf2 in melanocytes in the foreskins of neonates and 2 people with stable vitiligo.7 In contrast, glycerin, which is a natural component of licorice rhizomes and roots, reduces oxidative stress through activation of Nrf2 and increased expression of hemooxygenase 1 in macrophages.1 Berberine is a natural isoquinoline alkaloid with antioxidant activity that acts by activating the Nrf2-ARE pathway (Table 2).2
Description of studies of effects of molecules on Nrf2 function.
| Author/year | Country | LE/SR | Type of study | Contribution |
|---|---|---|---|---|
| Mou et al.,1 2019 | China | NA | Molecular biology study | Glycyrrhizin as a protector against oxidative stress in melanocytes through activation of the Nrf2 pathway |
| Jiang et al.,2 2019 | China | NA | Molecular biology study | Berberine as an inducer of Nrf2 nuclear translocation and total Nrf2 levels in blood |
| Ma et al.,8 2018 | China | NA | Molecular biology study | What is baicalein and its upregulation in the Nrf2 signaling pathway |
| Arowojolu et al.,10 2017 | United States | NA | Molecular biology study | Action of 4-TBP and MBEH on Nrf2 (increase of Nrf2 following oxidative stress produced by 4-TBP and MBEH) |
| Kim et al.,11 2017 | South Korea | 4/C | Longitudinal | 4-TBP and HQ causing decrease in activation of the Nrf2 pathway |
| Ben-Yehuda Greenwald et al.,12 2017 | Switzerland | NA | Molecular biology study | Iodine and iodide as activators of Nrf2 pathway |
| Tsuji et al.,18 2017 | Japan | NA | Molecular biology study | PAPLAL as an inducer of Nrf2 nuclear translocation |
| Jung et al.,19 2017 | Korea | NA | Molecular biology study | Afzelin as an activator of the Nrf2-ARE signaling pathway in vitro |
| Chang et al.,20 2017 | China | NA | Molecular biology study | High doses of simvastatin as an Nrf2 activator |
| Vanderweil et al.,21 2017 | United States | 1b/A | Clinical trial | Simvastatin for the treatment of vitiligo |
| Fang et al.,22 2020 | China | NA | Molecular biology study | Molecular hydrogen as activator of the Nrf2-ARE signaling pathway |
| Yang et al.,23 2020 | China | NA | Molecular biology study | Ginger extract as an activator of the Nrf2 signaling pathway |
| Yuan et al.,24 2020 | China | NA | Molecular biology study | Derivative of the Paeonia lactiflora plant as an activator of the Nrf2 signaling pathway |
| Zhang et al.,25 2020 | China | NA | Molecular biology study | Extract of plant origin as an activator of the Nrf2 |
| Du et al.,26 2021 | China | NA | Molecular biology study | Folic acid and its action on the Nrf2 signaling pathway |
| Zhang et al.,27 2019 | China | NA | Molecular biology study | Ginkgo biloba extract as an activator of the Nrf2-ARE signaling pathway |
Abbreviations: ARE, antioxidant response elements; HQ, hydroquinone; MBEH, monobenzyl ethyl hydroquinone; LE/SR, level of evidence and strength of recommendation according to the Centre for Evidence-Based Medicine de Oxford scale; NA, not applicable as it meets one of the 11 Bradford Hill causality criteria (biological plausibility in basic research); Nfr2, nuclear factor resembling erythroid factor 2; PAPLAL, palladium and platinum nanoparticle solution; 4-TBP: 4-tertiaryl butyl phenol.
Metal nanoparticles such as those comprised of palladium have antioxidant catalytic activity, and so an emulsion of the 2 substances was developed by investigators in Japan. This product, used for the treatment of vitiligo and skin aging, acts by inducing Nrf2 translocation to the nucleus with upregulation of the Nrf2-NADH quinin oxidoreductase 1 pathway and, in a secondary action, by inducing production of superoxide dismutase 1.18
Other substances tested in human epidermal melanocytes from patients with vitiligo include flavonoids; an example is afzelin, which has been shown to significantly inhibit hydrogen-peroxide induced cell death, ROS production, and peroxidation of lipids in melanocytes, leading to deactivation of the glycogen synthase kinase 3 beta pathway and allowing greater activation of the Nrf2-ARE pathway.19
In 2004, a patient with vitiligo and hypercholesterolemia was reported to experience rapid repigmentation of the skin after receiving high doses of simvastatin. Recently, a randomized clinical trial conducted with oral simvastatin in patients with nonsegmental vitiligo who had not benefitted from prior treatment did not demonstrate any clinical benefit. In contrast, in vitro studies have shown that simvastatin can be a useful molecule for activating the cell autophagy mechanism, improving cell viability, and enhancing the activity of antioxidant enzymes.20 It is likely that the discrepancy between in vivo and in vitro studies are the result of the limited dosing of simvastatin in humans due to the risk of complications such as rhabdomyolysis. Another study found that atorvastatin along with UVB phototherapy can be useful for reducing lesion propagation in patients with active vitiligo. The study outcomes were dose-dependent.21
Some phytochemicals such as cinnamaldehyde extract have been reported as stimulators of Nrf2 translocation and upregulators of hemooxygenase 1 expression in HaCaT cells, a keratinocyte cell line, through oxidative or covalent modification of the cytosolic repressor Keap1, or by Nrf2 phosphorylation.9
A more recent study assessed the effect of molecular hydrogen on the Nrf2-ARE pathway in cells under oxidative stress.22 This molecule was found to be able to drastically regulate expression of cytosolic and nuclear Nrf2, as well as to promote translocation of Nrf2 to the nucleus and to increase expression of Nrf2 target genes. The authors concluded that molecular hydrogen can act as an antioxidant in melanocytes by promoting activation of the Nrf2-ARE pathway.
Currently, new herb extracts with apparent antioxidant activity are under study. For example, 6-shogaol, an active component of ginger, has been shown to be useful for protecting melanocytes from oxidative stress caused by hydrogen peroxide through activation of the Nrf2 pathway and increased expression of this pathway.23 Another plant derivative is paeoniflorin, which is a chemical compound derived from the plant Paeonia lactiflora. This compound seems to be able to reduce cell apoptosis, improve cell viability, and activate the Nrf2 factor pathway, among other functions.24 Apigenin, an aglycone of plant origin, has also been studied as a regulator of oxidative stress.25 Recently, folic acid has been reported as an effective activator of the Nrf2 pathway.26
Other compounds that could activate Nrf2 include plant sterols, carbonitriles, dietary supplements that contain ellagic acid or Ginkgo biloba, treatment with iodine solution, and certain drugs such as ketoconazole.9,12,27
ConclusionsNrf2 is essential for maintaining cell redox by promoting phase ii antioxidant enzymes. Several variations in cell localization, structure, and function have been reported for this transcription factor, which may be closely linked to onset of diseases such as vitiligo. It is important to investigate its function and how this transcription factor can be stimulated, as such knowledge could lead to new therapeutic options for patients with vitiligo. Current evidence is not strong enough to establish recommendations; however, knowledge of this transcription factor may contribute to our understanding of the disease and point to potential future treatments.28
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank Dr. Martha Morales, head of Teaching and Research and Dr. Fermín Jurado Santa-Cruz, director of the Dermatology Center Dr. Ladislao de la Pascua, for their support in the drafting of this article.