Mpox is an emerging zoonotic disease that has spread rapidly around the world. It has been declared a public health emergency of international concern by the World Health Organization. This review is an update for dermatologists on the epidemiology, clinical presentation, diagnosis, and treatment of Mpox. The primary mode of transmission in the current outbreak is close physical contact during sexual activity. Although most of the initial cases were reported in men who have sex with men, anyone who has close contact with an infected person or contaminated fomites is at risk. Classic prodromal features of Mpox include subclinical manifestations and a mild rash. Complications are common but rarely require hospitalization. Polymerase chain reaction analysis of mucocutaneous lesions is the test of choice for a definitive diagnosis. In the absence of specific treatments, management focuses on symptomatic relief.
MPOX es una enfermedad zoonótica emergente que se ha propagado rápidamente por todo el mundo y que ha sido declarada por la OMS como una emergencia de salud pública de interés internacional. Esta es una revisión no sistemática de los aspectos clínicos, epidemiológicos, diagnósticos y terapéuticos más relevantes para el dermatólogo. En el brote actual, la transmisión durante las relaciones sexuales es la principal forma de contagio como resultado del contacto físico cercano. Si bien los casos iniciales se informaron en hombres que tienen sexo con hombres, cualquier persona en contacto cercano con personas o fómites infectados está en riesgo. El pródromo clásico puede ser subclínico y la erupción puede ser sutil. Las complicaciones son frecuentes, pero el requerimiento de hospitalización es infrecuente. El diagnóstico definitivo se realiza mediante PCR de las lesiones mucocutáneas. Actualmente no existen tratamientos específicos, y el tratamiento sintomático es el pilar terapéutico.
Mpox (formerly monkey pox) is caused by an orthopoxvirus1 from the same genus as the Variola virus (responsible for smallpox) and the Vaccinia virus (used in the smallpox vaccine).2 With the eradication of smallpox, Mpox became the most significant and pathogenic orthopoxvirus in humans.
History and EpidemiologyMpox was first isolated in Denmark from a colony of Cynomolgus monkeys transported from a Singapore laboratory for polio virus research.1 Its ability to cause disease in humans was first recognized in 1970 during intensive surveillance in the Democratic Republic of the Congo (DRC) to ensure the eradication of smallpox.3 These early studies showed that the smallpox vaccination provided 85% protection against Mpox.4
Since then, Mpox has mainly been observed in central and western Africa, where it is endemic. Many strains of Mpox and 2 clades of the virus – the Congo Basin clade (clade 1) and the West African clade (clade 2) – have been identified and sequenced.5,6
The increase in Mpox cases in Africa since 2016 has been particularly notable in the DRC, Nigeria, the Central African Republic, Liberia, and Sierra Leone.7 Prior to the current outbreak, the largest outbreak in humans outside the DRC occurred in Nigeria in 2017 and 2018.6
The first cases of Mpox in humans outside Africa were reported in several US states between May and June 2003, with 47 confirmed or probable cases.8 Most were zoonotic and had been caused by contact with infected prairie dogs. Person-to-person spread was not reported, and the likely source of infection was considered to be indirect contact.9
In February 2022, Bunge et al.10 published a systematic review on the changing epidemiology of Mpox in humans that dramatically illustrated the progressive rise in cases.11–13
A few months later, on May 7, 2022, a case of Mpox involving a UK citizen who had returned from Nigeria was confirmed in England.14 The outbreak spread rapidly, with several countries in the European Union reporting cases not linked to endemic areas.15
On July 23, 2022, the World Health Organization (WHO) declared the 2022 Mpox outbreak a public health emergency of international concern.16
Transmission Route and ContagionAs a zoonotic disease, Mpox is acquired through contact with an infected animal (body fluids, bites, or ingestion of bush meat).17 The reservoir is unknown, but it is likely to be rodent.18 Viral particles can enter the body through broken skin, the respiratory tract, or mucosal surfaces (e.g., mouth, nose, vagina, anus, conjunctiva).19,20 Evidence of Mpox infection has been found in many types of animals in Africa (squirrels, rats, African dormouse, and monkeys), but like humans, these are incidental hosts.
Person-to-person spread can occur in several ways,19,20 namely via
- •
direct contact: contact with wounds, scabs, or infectious body fluids, facilitated by mucosal microabrasions
- •
indirect contact through fomites
- •
respiratory secretions: through microdroplets during prolonged close contact
- •
vertical transmission: through the placental barrier, causing congenital Mpox
Infections caused by orthopoxviruses can be classified as systemic or localized depending on the route of entry.20–22 Systemic disease following a respiratory infection usually manifests as a diffuse rash, viremia, and spread of the virus to internal organs. Subcutaneous inoculation, by contrast, may cause a localized rash at the site of entry, with involvement of the regional lymphatic system, followed or not by a few disseminated lesions.
Prior to the 2022 outbreak, transmission outside the family environment and sustained person-to-person spread were rare. The suspected route of transmission in 95% of patients in the outbreak starting in 2022, however, was sexual contact.23 Between 23% and 26% of patients had a regular sexual partner with Mpox,23–25 compared with between 1% and 3% who had been exposed to a contact in the home environment.24,26 Just 1% of patients had been infected as the result of an occupational contact (health care provider).24 In most cases, it was difficult to conduct full contact tracing and identify the source of the infection.
Mpox DNA has been detected in saliva, rectal, nasopharyngeal, semen, urine, and fecal samples, suggesting that body fluids might have an important role in transmission.27
One study of viral loads in different locations found higher loads in skin versus pharyngeal swabs,26 suggesting that direct contact is the main transmission route in the current outbreak, with respiratory secretions playing a lesser role. Few asymptomatic infections have been described,28 and in these cases, it was not actually possible to confirm whether the patients were completely asymptomatic or just paucisymptomatic.
A person is considered to be infectious from the onset of symptoms to re-epithelialization of all lesions, a process that can last 2 to 4 weeks or more. Persistent positivity (>3 weeks) has been demonstrated by polymerase chain reaction (PCR) in blood and upper respiratory tract samples,29 but the significance of these findings in terms of infectivity is unclear. Based on studies of replication-competent viruses in some studies,30,31 infectivity time might actually be shorter.
Clinical ManifestationsEndemic FormAffected PopulationHistorically, children have been affected most by Mpox. Against an increasing number of cases in the DRC in the 1980s, patients vaccinated against smallpox were found to have a milder rash and less swollen lymph nodes, and there were no associated deaths.32,33
Clinical CharacteristicsIncubation time, which varies between 5 and 13 days (range, 4–21 days)9 could be shorter after invasive exposure (animal bites or scratches) compared with noninvasive exposure (touch).9
Mpox has traditionally caused systemic disease, which can be divided into 2 periods: an invasion period and a rash period.34
Invasion period. In endemic areas, where the main route of transmission is respiratory, the invasion period lasts from 0 to 5 days. Systemic symptoms are common and precede the rash (prodromal stage) or occur shortly afterwards (early clinical stage). Symptoms described include6,17,18,20
- •
fever (88%)
- •
intense headache (79%)
- •
swollen lymph nodes (69%):
- ∘
inguinal and cervical
- ∘
distinctive feature in relation to other diseases (chickenpox, measles, smallpox).
- ∘
- •
myalgia (63%)
- •
odynophagia (58%)
- •
profound weakness or fatigue
Skin rash period. This usually starts within 1 to 3 days of fever onset.35 In endemic areas, the rash tends to affect the
- •
face (96%)
- •
legs (91%)
- •
trunk (80%)
- •
arms (79%)
- •
palms (69%)
- •
genitalia (68%)
- •
soles (64%)
Lesion numbers vary from several to thousands,32 although the mean count at presentation has been estimated at 370.6
Sequelae, Severity, and Case FatalityBlood tests show multiple nonspecific findings, such as abnormal aminotransferases, leukocytosis, thrombocytopenia, and hypoalbuminemia.33
Severe Mpox is more common among children and is related to
- •
extent of virus exposure
- •
health status
- •
complications
- •
access to quality healthcare
Data on disease severity in immunocompromised patients is scarce. In previous outbreaks, advanced HIV infection was linked to an increased risk of severe disease, with more and longer-lasting lesions.33
The most common complications are pain, ulceration, and bacterial superinfection. Complications affecting other organs have also been described, such as meningoencephalitis, myocarditis, bronchopneumonia, and kidney failure. Neurological involvement and sepsis due to bacterial superinfection have the highest mortality risk.19 Similarly to in smallpox, the most common sequelae in Mpox are scars.
Historically, the case fatality rate in Africa has been 1% to 10% (depending on the clade), although the figure has lowered in recent years (3% to 6%).19
Clinical Characteristics During the 2022 OutbreakSeveral series have been published since the start of the 2022 outbreak.23–26,36,37 The data are very similar across the studies and show features that differentiate this outbreak from previous ones. The median incubation period has been estimated at 6 to 9 days (range, 4–10 days).24,26,38,39
Affected PopulationThe current outbreak predominantly affects men who have sex with men (MSM), with a median age of 38 years. It should be noted, however, that the mode of community transmission may change. Case rates are very high among patients with (generally well-controlled) HIV coinfection, and high rates have also been reported for HIV-negative people on pre-exposure prophylaxis (PrEP).23–26,36,37
Risk factors for sexually transmitted infections (STIs) are present in a high proportion of patients, with over 50% of Mpox patients who underwent STI screening testing positive for at least 1 STI.24,25
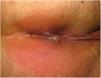
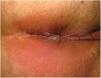
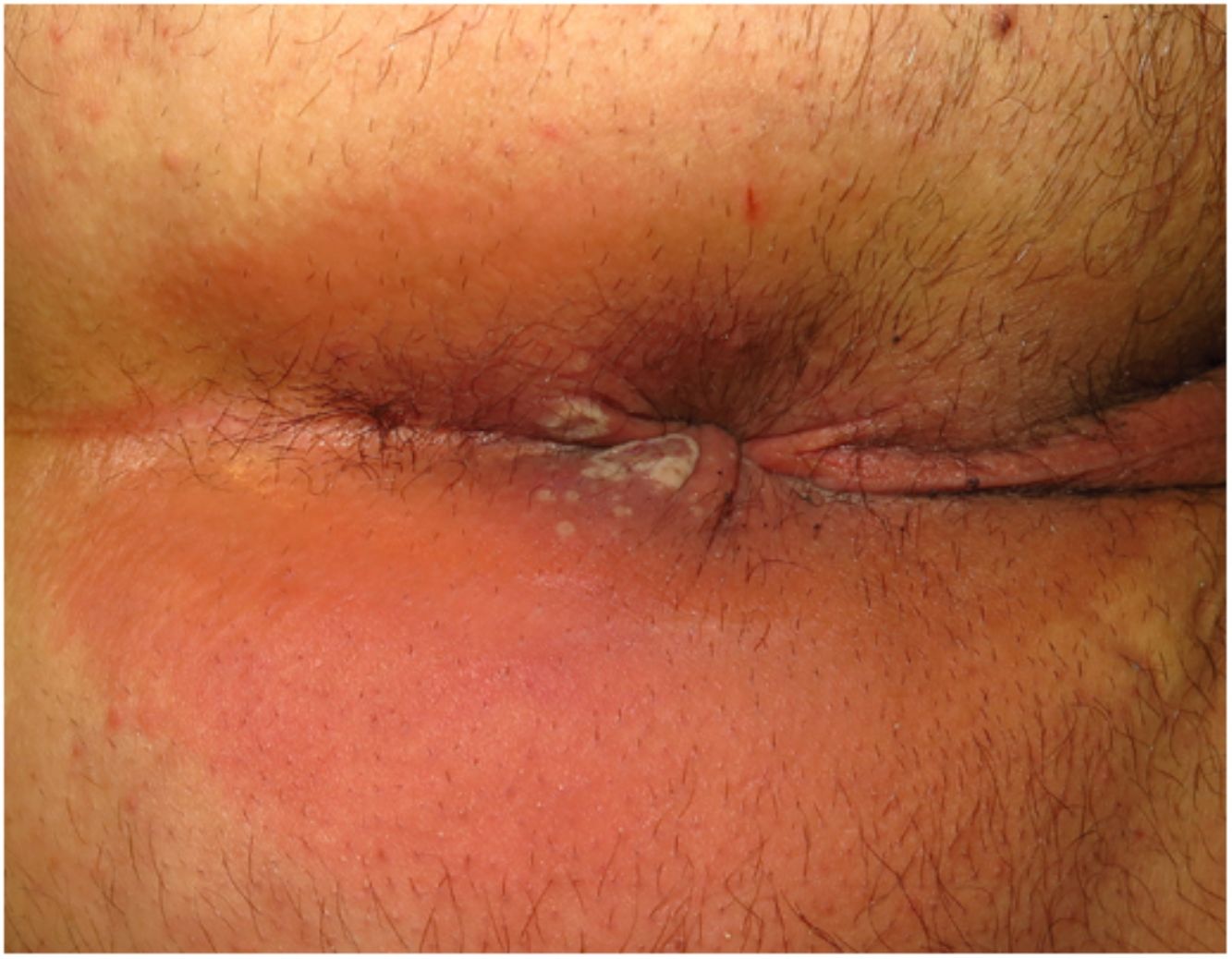
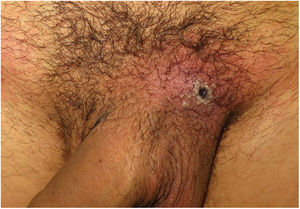
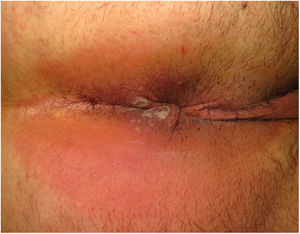
Clinical CharacteristicsSimilarly to with other poxviruses (orf and molluscum), primary lesions in inoculated areas are usually pseudopustules (solid papules that look like pustules but in which it is impossible to scrape the roof and obtain pus) (Fig. 1).24 These findings have been confirmed histologically.40 Inoculation lesions vary in number and are mainly observed in the genital or perianal regions (Fig. 2) or on the face24 (Table 1). The lesions are usually well-circumscribed, umbilicated, deep lesions that are described as painful and take weeks to heal completely. In some cases, they have a tendency to coalesce or become necrotic, and hence become ulcerated. They are usually accompanied by swollen regional lymph nodes and surrounding edema, which in some cases can be very severe.
Summary of Main Characteristics of Mpox Cases Published in the 2022 Outbreak.
| Study | N Engl J MedThornhill et al.23 | BMJPatel et al.25 | LancetTarín-Vicente et al.26 | Br J DermatolCatalà et al.24 | Clin Microbiol InfectMailhe et al.37 |
|---|---|---|---|---|---|
| No. | 528 | 197 | 181 | 185 | 264 |
| Sex | Male > 99%Transgender < 1% | Male 100%Female 0% | Male 97%Female 3% | Male 100%Female 0% | Male 99%Female 1%Transgender 1% |
| Mean age, y | 38 | 38 | 37 | 38.7 | 35 |
| Sexual orientation | MSM or 99%bisexualHeterosexual 1% | MSM or > 99%bisexual | MSM or 92%bisexualHeterosexual 8% | MSM or 99%bisexualHeterosexual 1% | MSM 95% |
| HIV | 41%Viral load < 50copies/mL 95%ART therapy 96% | 35.9%Viral load < 50copies/mL 78.6%ART therapy 91.4% | 40%CD4 > 500cells/μL 89%ART therapy 99% | 42%Viral load < 50copies/mL 92% | 29% |
| If HIV(−)PrEP user | 57% | − | − | 77% | 71% |
| History of smallpox vaccination | 9% | − | 18% | 11% | 12% |
| Cutaneous lesions, No. (%) | < 5 (39%)5–10 (25%)11–20 (21%)> 20 (11%) | 1 (11.2%)2–10 (51.8%)11–50 (18.3%)51–100 (0%)≥ 100 (4.1%) | 1–2 (12%)3–20 (80%)> 20 (8%) | 1 (11%)2–25 (82%)26–100 (6%)> 100 (1%) | Mean 5Range, 3–10 |
| Extracutaneous manifestations | |||||
| Swollen lymph nodes | 56% | 57.9% (mostly inguinal or cervical) | 85% (mostly inguinal or cervical) | 56% | 69% |
| Fever | 62% | 61.9% | 72% | 54% | 68% |
| Weakness or fatigue | 41% | 23.4% | 81% | 44% | – |
| Myalgia | 31% | 31.5% | 44% | – | |
| Headache | 27% | 24.8% | 53% | 32% | 35% |
| Odynophagia | 21% | 16.8% | 36% | 18% | 20% |
Abbreviations: ART, antiretroviral therapy; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis.
Other forms of presentation include perioral pseudopustules, lesions on the tongue–which are usually white papules with a central depression–or ulcerated lesions of the oral mucosa or lips. Solitary primary lesions forming a whitlow have also been observed on the fingers.24
This first phase may be followed by a secondary rash with lesions that progress from macules to vesicles, pustules, and scabs. (This need not occur simultaneously.) These lesions are described as pruritic and take days to resolve. They are located on the face, scalp, arms, legs, palms, soles, and trunk. Although extracutaneous manifestations are very common24–26,36,37 and generally occur in association with cutaneous manifestations, just 36% of patients report prodromal symptoms before the onset of skin lesions.24 Between 6% and 13.7% of patients develop a maculopapular morbilliform rash,24,25 which may be due to an immune phenomenon analogous to that of other viral infections, such as those caused by Epstein-Barr virus, cytomegalovirus, and SARS-CoV-2. Depending on the context, however, other possibilities such as a toxic drug reaction and secondary syphilis must be ruled out.
Mucocutaneous Complications, Severity, and Case FatalityMpox is mostly a mild, self-limiting, disease that resolves in 1 to 4 weeks. Some patients, however, may develop serious complications or illnesses.
Proctitis. Between 14% and 36% of patients experience anorectal pain, tenesmus, constipation, purulent discharge, or bleeding,23–26,36,37 accompanied or not by visible lesions in the perianal area. These patients usually have a history of receptive anal intercourse and more frequently develop a systemic prodrome.26 Proctoscopy is not recommended given the intense associated pain. Magnetic resonance imaging is preferable when rectal perforation is suspected (intense pain or signs of sepsis).25 Other causes of proctitis must always be ruled out.
Ulcerative tonsillopharyngitis. Odynophagia and dysphagia have been described in between 10% and 21% of patients.22–26,36,37 They occur in association with nonspecific ulcerative plaques on the palatine tonsils or pharynx and are generally accompanied by painful, swollen cervical lymph nodes. Mpox should be ruled out in areas with high transmission rates and in patients with epidemiological risk factors and a negative rapid antigen test for group A Streptococcus.26
Genital edema. Lesions on the penis, especially on the penile glans, foreskin, and urinary meatus, may be associated with glans and foreskin swelling that can cause paraphimosis (8%–16% of reported cases).22–26,36,37 Urologic evaluation may be required.
Severe bacterial superinfection. Progressive swelling, pain, and worsening ulcerative lesions can indicate a secondary bacterial infection that may require hospitalization and intravenous treatment.25 This complication was reported in 3%–4% of cases in the 2022 outbreak.22–26,36,37 Sample collection for culture and antibiogram is recommended, as multiresistant bacteria have been detected.25
Eye involvement. Eye involvement in the form of conjunctivitis, blepharitis, eyelid papules or edema, corneal ulcers, and/or periorbital cellulitis has been reported.41 Corneal involvement in particular can compromise vision and requires a rapid response in collaboration with the ophthalmology department.
Hospitalization was uncommon during the 2022 outbreak (1–13% of all reported cases). The main reason for admission was intravenous treatment for pain control. Other reasons were management of complications (secondary infections, penile edema, dysphagia, encephalitis, ocular disease, myocarditis) or proper isolation.22–26,36,37 Case fatality rates in western countries have been very low (<0.1%) compared with those reported for African outbreaks.42
Similarly to in the 2003 US outbreak,33 prior smallpox vaccination was not associated with symptom severity.22–26 Severity also did not differ between patients with or without HIV infection. It should be noted, however, that most patients with HIV coinfection in the 2022 outbreak were receiving effective antiretroviral therapy and are a well-controlled population, unlike previous cases involving patients with advanced-stage HIV.33,43
DiagnosisMpox is diagnosed on the basis of epidemiological, clinical, and laboratory findings. The case definitions proposed by the European Centre for Disease Prevention and Control (ECDC) are provided in Table 2.44
Criteria for Suspected, Probable, and Confirmed Cases According to the Criteria of the European Centre for Disease Prevention and Control.40
| Suspected case |
| (a) A person who is a contact of a probable or confirmed Mpox case in the 21 days before the onset of signs or symptoms, and who presents with any of the following: acute onset of fever (>38.5°C), headache, myalgia (muscle pain/body aches), back pain, profound weakness or fatigueOR(b) A person presenting since January 1, 2022 with an unexplained acute skin rash, mucosal lesions, or swollen lymph nodes. The skin rash may include single or multiple lesions in the anogenital region or elsewhere on the body. Mucosal lesions may include single or multiple oral, conjunctival, urethral, penile, vaginal, or anorectal lesions. Anorectal lesions can also manifest as anorectal inflammation (proctitis), pain, and/or bleeding.And for which other causes have been ruled out: varicella zoster, herpes zoster, measles, herpes simplex, bacterial skin infections, disseminated gonococcus infection, primary or secondary syphilis, chancroid, lymphogranuloma venereum, granuloma inguinale, molluscum contagiosum, allergic reaction (e.g., to plants), and any other locally relevant common causes of papular or vesicular rash |
| Probable case |
| A person presenting with an unexplained acute skin rash, mucosal lesions, or swollen lymph nodes. The skin rash may include single or multiple lesions in the anogenital region or elsewhere on the body. Mucosal lesions may include single or multiple oral, conjunctival, urethral, penile, vaginal, or anorectal lesions. Anorectal lesions can also manifest as anorectal inflammation (proctitis), pain and/or bleeding.AND1 or more of the following:1. has an epidemiological linka to a probable or confirmed case of Mpox in the 21 days before symptom onset2. identifies as gay, bisexual, or other cisgender or transgender man who has sex with men3. has had multiple and/or casual sexual partners in the 21 days before symptom onset4. has detectable levels of anti-orthopoxvirus immunoglobulin (Ig) M antibodyb (during the period of 4–56 days after rash onset) or a 4-fold rise in IgG antibody titer based on acute (up to day 5–7) and convalescent (day 21 onwards) samples in the absence of a recent smallpox/Mpox vaccination or other known exposure to orthopoxvirus5. has a positive test result for orthopoxvirus infection (e.g., orthopoxvirus-specific polymerase chain reaction [PCR]) without Mpox-specific PCR or sequencingc |
| Confirmed case |
| A person with laboratory-confirmed Mpox infection by detection of unique sequences of viral DNA by PCRc and/or sequencing |
The person has been exposed to a probable or confirmed Mpox case. A contact is defined as a person who has been exposed to an infected person during the infection period, i.e., the period beginning with the onset of the index case's first symptoms and ending when all scabs have fallen off, and who has 1 or more of the following exposures with a probable or confirmed case of Mpox: a) direct skin-to-skin and skin-to-mucosal physical contact (such as touching, hugging, kissing, and intimate or sexual contact); b) contact with contaminated materials such as clothing or bedding, including material dislodged from bedding or surfaces during handling of laundry or cleaning of contaminated rooms; c) prolonged face-to-face respiratory exposure in close proximity; d) respiratory exposure (i.e., possible inhalation of) or eye mucosal exposure to lesion material (e.g., scabs/crusts) from an infected person; e) the former also apply for health workers potentially exposed in the absence of proper use of appropriate personal protective equipment.
Serology can be used for retrospective case classification for a probable case in specific circumstances such as when diagnostic testing through PCR of skin lesion specimens has not been possible, or in the context of research with standardized data collection. The primary diagnostic test for Mpox diagnosis is PCR of skin lesion material or another specimen such as an oral or nasopharyngeal swab as appropriate. Serology should not be used as a first-line diagnostic test.
The following entities should be entertained in the differential diagnosis: chickenpox, herpes zoster infection, measles, Zika virus infection, dengue, chikungunya, herpes simplex, impetigo, methicillin-resistant Staphylococcus aureus infection, disseminated or localized gonorrhea, primary or secondary syphilis, chancroid, lymphogranuloma venereum, and molluscum contagiosum.
Patients who meet the criteria for a suspected or probable case of Mpox should undergo specific PCR testing for this or other orthopoxviruses, which should then be confirmed by sequencing. If negative, these patients should be excluded.
Sequencing of genomes obtained in Spain showed that they belong to the West African clade and are almost identical to genomes uploaded in other European countries.45
Isolation, Quarantine, and Contact TracingPatients who undergo diagnostic PCR should remain isolated until results are available. Contact and respiratory isolation must be maintained throughout the infectious period. Respiratory isolation is an empirical precaution, and some authors have speculated that the degree of viremia following inoculation is low or absent, meaning that there would be minimal replication in the respiratory tract and little or no transmission through respiratory droplets.26
Mpox is a notifiable disease. Contact tracing, even if incomplete, can help reduce the spread of the virus.46 Quarantine for asymptomatic contacts following exposure to Mpox should be considered on a case-by-case basis.
Information on transmission during the 2022 outbreak is emerging rapidly. It is unknown whether viral DNA detected in samples from asymptomatic individuals is replication competent. One study conducted in Israel highlighted the strong correlation between Mpox quantification cycle (Cq) levels and virus infectivity and defined a threshold for predicting slightly infectious or noninfectious specimens (Cq ≥35; viral DNA ≤4300copies/mL).47
TreatmentCurrently, there are no treatments specifically approved for Mpox by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Symptomatic treatment thus is the mainstay option and consists of15
- •
antipyretics
- •
nonsteroidal anti-inflammatory drugs
- •
topical corticosteroids (or systemic when there is significant inflammation)
- •
painkillers
- •
creation of a clean, moist microenvironment with covering of infectious ulcers to potentially mitigate transmission and favor re-epithelialization of the exanthem
- •
appropriate antibiotics to treat secondary bacterial infections
Antivirals approved for the treatment of smallpox may be prescribed.
- •
Tecovirimat is the only antiviral with EMA approval for the treatment of orthopoxvirus infections, including Mpox.
- •
Brincidofovir has FDA but not EMA approval for the treatment of Mpox.
- •
Cidofovir has demonstrated in vitro activity against smallpox, but nephrotoxicity associated with systemic use makes it unsuitable as a first-line treatment. Topical off-label use, however, does seem to be associated with a shorter time to resolution of lesions and better cosmetic outcomes.48
The risks and benefits of initiating specific treatments should be weighed up and local guidelines followed.49
VaccinesSmallpox vaccination may provide cross-protection of up to an estimated 85% against Mpox.4 This protective effect diminishes with time (>20 years), although lifelong protection against severe disease might be provided through B and T memory cells.50
Early off-label smallpox vaccination (within 4 days of exposure) may prevent Mpox or reduce its severity.50
A third-generation, nonreplicating vaccine (Imvanex, modified vaccinia virus Ankara) has been licensed for use against smallpox and been recommended for people at risk51,52:
- •
People who engage in high-risk sexual practices, mainly but not exclusively MSM on PrEP or with HIV infection, and
- •
People with occupational risk, such as health personnel in STI/HIV clinics, laboratory staff handling potentially contaminated samples, and personnel in charge of cleaning surfaces in specialized clinics.
Previous-generation smallpox vaccines (Dryvax and ACAM2000) are no longer authorized in the European Union due to their adverse effects.
The standard regimen involves a 0.5-mL subcutaneous injection (2 doses with a 28-day interval). Against a background of vaccine shortages and global demand, the EMA approved an alternative intradermal regimen of 0.1-mL (2 doses with a 28-day interval) on August 19, 2022.53
ConclusionsMpox is an emerging zoonotic disease that has spread rapidly around the world. There is concern that its continued spread will lead to new endemic areas.
Sexual contact was the main route of transmission during the 2022 Mpox outbreak. Although the earliest cases were primarily reported in MSM, anyone in contact with infected people, animals, or contaminated fomites is at risk of developing the disease within 21 days.
Systemic prodromal features may be subclinical, and the rash (which can be mild) may start in the genital or anal regions. Typical pseudopustules and swollen lymph nodes are useful findings for distinguishing Mpox from other diseases.
Local complications are common though not generally serious.
A definitive diagnosis of Mpox requires PCR detection of viral DNA in a lesion sample. STI coinfection is possible and common.
Conflicts of InterestThe authors declare that they have no conflicts of interest.