Atypical fibroxanthoma and pleomorphic dermal sarcoma (PDS) are rare mesenchymal tumors. Due to the low incidence of PDS and a historically confusing nomenclature, little is known about the true aggressiveness of this tumor. The aim of this study was to investigate clinical and histologic risk factors for recurrence in PDS.
Material and methodsRetrospective, observational, bicentric study of 31 PDSs diagnosed and treated at Hospital Clínico Universitario de Valencia and Instituto Valenciano de Oncología in Valencia, Spain, between 2005 and 2020. We described the clinical and histologic features of these tumors and performed univariate analysis and multivariate Cox regression analysis.
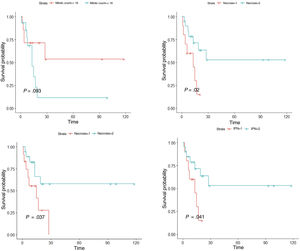
ResultsIn the univariate analysis, tumor recurrence (P<.001), necrosis (P=.020), lymphovascular invasion (P=.037), perineural invasion (P=.041), and mitotic count (<18 vs ≥18 mitoses per 10 high-power fields) (P=.093) were associated with worse disease-free survival. In the multivariate Cox regression analysis, mitotic count and lymphovascular invasion retained their significance as predictors of worse disease-free survival (P<.05).
ConclusionsPDS is an aggressive tumor in which a high mitotic count (≥18) and lymphovascular invasion are associated with a higher risk of recurrence and worse disease-free survival. Necrosis and perineural invasion are also probably linked to increased tumor aggressiveness.
El fibroxantoma atípico (FXA) y el sarcoma pleomórfico dérmico (SPD) son neoplasias de origen mesenquimal poco frecuentes. Debido a la baja incidencia del SPD y a una nomenclatura históricamente confusa, existe poca información acerca de la verdadera agresividad de este tumor. Realizamos el presente estudio con el objetivo de identificar qué características clínicas y/o histológicas del SPD son predictoras de riesgo de recidiva.
Material y métodoSe diseñó un estudio bicéntrico observacional retrospectivo de 31 casos de SPD diagnosticados y tratados en el Hospital Clínico Universitario de Valencia y el Instituto Valenciano de Oncología, entre los años 2005 y 2020. Se realizó un análisis descriptivo de las características clínicas e histológicas, un análisis inferencial univariado y un análisis multivariado mediante la regresión de Cox.
ResultadosEn el análisis univariado, la recidiva tumoral (p<0,001), la necrosis (p=0,020), la infiltración linfovascular (p=0,037), la infiltración perineural (p=0,041) y el número de mitosis (categorizado en <18 y ≥18 por 10 campos de gran aumento) (p=0,093), se asociaron a una menor supervivencia libre de enfermedad. En el análisis multivariado, el número de mitosis (categorizado en <18 y ≥18) y la infiltración linfovascular (p<0,05), se asociaron a una menor supervivencia libre de enfermedad.
ConclusiónEl SPD es un tumor agresivo, en el que la presencia de un alto recuento mitótico (≥18) y/o invasión linfovascular se asocian a un mayor riesgo de recidiva y a una peor supervivencia libre de enfermedad. La necrosis y la infiltración perineural, también son hallazgos que probablemente se asocien a una mayor agresividad tumoral.
Atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS) are rare mesenchymal tumors.1–4 Both arise in the sun-exposed skin of older people and are characterized by a dermal proliferation of spindle and epithelioid cells with a high mitotic count and pronounced pleomorphism.2,5,6 While AFX is clinically benign, PDS may exhibit local aggressive behavior and has the potential to metastasize.2–4 The classification of these tumors has been historically confusing due to changing, imprecise terminology, with the terms AFX and malignant fibrous histiocytoma being used interchangeably and even together.2,4,7,8 In 2012, the term PDS was coined to describe skin tumors without a clear line of cell differentiation and with general clinicopathological characteristics similar to those of AFX but with additional aggressive features on histology.2,7 Necrosis, lymphovascular invasion, perineural invasion, and/or evident subcutaneous invasion are hallmark histopathologic features of PDS, and at least 1 of them must be present for a diagnosis to be made.9AFX and PDS are considered to be entities on a common spectrum, with overlapping general epidemiologic and histologic characteristics but different clinical behaviors and aggressiveness.7,10,11 Some authors have even proposed using the terms AFX and, instead of PDF, AFX with high-risk histopathologic features.7 Due to the low incidence of PDS and a historically confusing nomenclature, little is known about the true aggressiveness of this tumor. Most studies to date have been descriptive, with just 2 recent series identifying potential prognostic factors. The aim of this study was to investigate clinical and histologic predictors of recurrence in PDS.
Material and MethodsRetrospective observational study of 31 cases of PDS diagnosed and treated at 2 hospitals (Instituto Valenciano de Oncología hospital and Hospital Clínico Universitario in Valencia, Spain) between 2005 and 2020. All data were collected from the patients’ medical records and the hospitals’ pathology databases. The following features were used to diagnose PDS: 1) presence of a mesenchymal tumor formed by pleomorphic spindle-shaped and/or epithelioid cells without clear cell differentiation in the immunohistochemical study (absence of expression for cytokeratins [CKAE1-AE3], S100 protein, desmin/caldesmon, or CD34), and 2) identification of at least 1 of the following histologic features: clear invasion of subcutaneous tissue, perivascular invasion, perineural invasion, and necrosis. These features were studied in hematoxylin-eosin–stained sections. Additional studies with smooth muscle actin, vimentin, CD10, CD68, and CD99 were performed in certain cases. Depth of invasion (Breslow depth) and number of mitoses per 10 high-power fields (×400) were also studied. Two dermatologists (Dr. Ríos-Viñuela and Dr. Pons Benavent) and 2 dermatopathologists (Dr. Traves and Dr. Sanmartín) reviewed all histologic samples from the Instituto Valenciano de Oncología. The samples at Hospital Clínico Universitario de Valencia were analyzed by Dr. Ríos-Viñuela and Dr. Pons Benavent. In this case, assessment of interobserver agreement between these dermatologists and a dermatopathologist (Dr. Monteagudo) showed an overall Cohen kappa of 0.7. All clinical and histologic features are shown in the supplementary material (Supplementary material 1; Supplementary Tables 1 and 2). The statistical analyses were conducted in IBM-SPSS 25.0 and included a descriptive analysis of clinical and histologic features, univariate inferential analysis (with Kaplan–Meier survival curves), and multivariate Cox regression analysis. A detailed description of the statistical analyses is provided in the supplementary material (Supplementary materials 1 and 2).
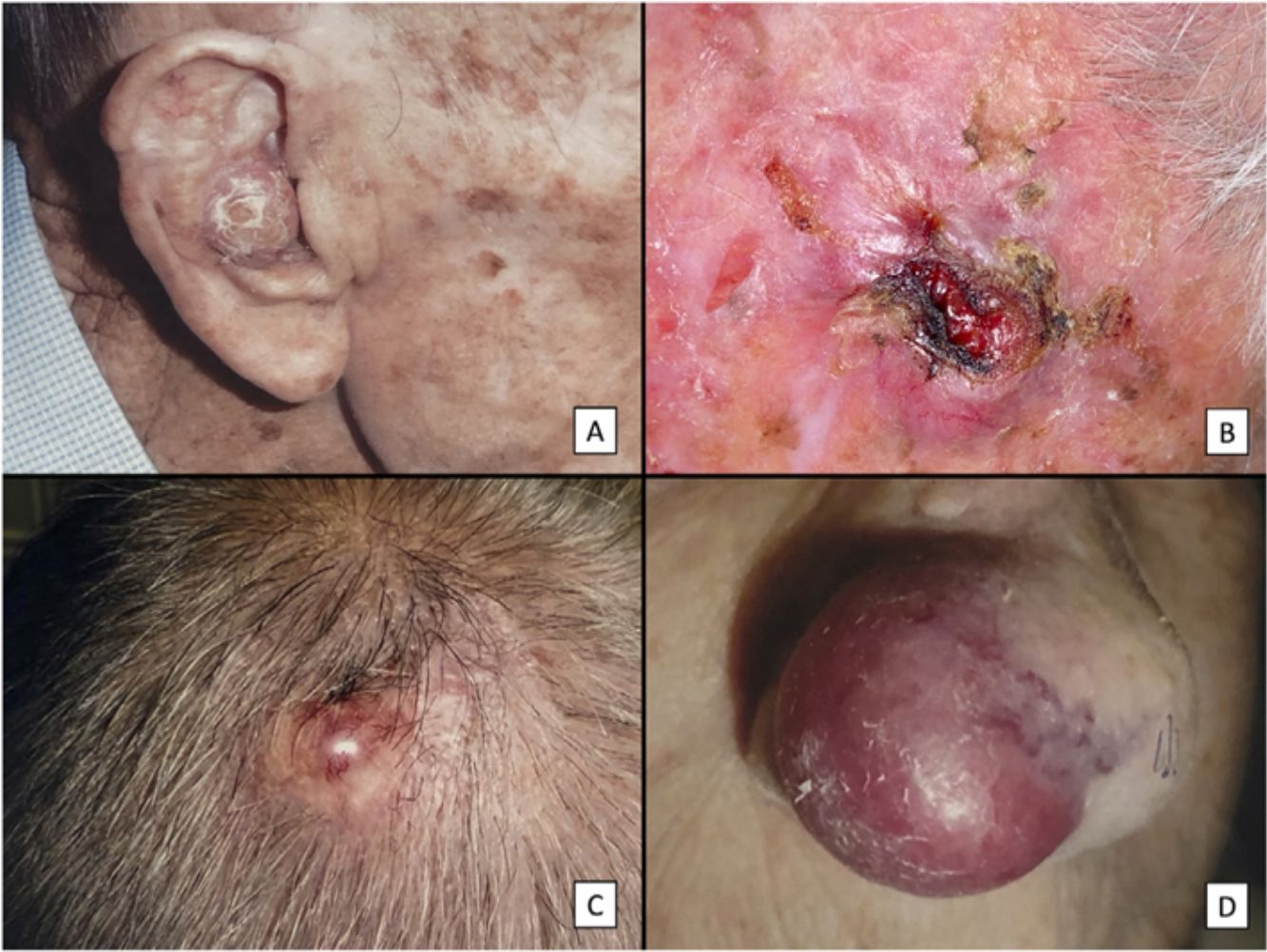
ResultsClinicopathologic VariantsWe studied 31 patients (3 women) with a median age of 82 years. Almost all of them (29, 93.5%) had a history of high chronic sun exposure and 23 (74.2%) had a history of nonmelanoma skin cancer other than PDS. Most PDSs were located on the scalp (22, 71%) (Fig. 1). The median largest diameter was 23mm and median time to diagnosis was 3 months. Nineteen patients (61%) had been initially diagnosed with AFX. All but 2 patients (treated by Mohs micrographic surgery) initially underwent conventional surgery, with clear margins obtained in 14 cases (45%).
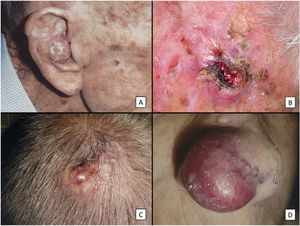
Clinical characteristics of pleomorphic dermal sarcoma. A, Large nodular tumor occupying the entire concha and invading the deeper layers. B, Ulcerated tumor with a fleshy, friable appearance on the scalp of a man with evident sun damage. C, Nodular tumor with prominent vascular features on the scalp of an older man. D, Large erythematous–violaceous tumor with prominent vascular features on the nasal ala of an older woman, with transmural invasion of the alar cartilage.
Five patients (16%) experienced 1 recurrence and 9 (29%) experienced 2 or more. Median time to the first recurrence was 7 months. Eight tumors (25%) were locally advanced (deep invasion, beyond the subcutaneous tissue) and 4 (13%) had metastasized to the lymph nodes, lungs, and/or brain. Median follow-up was 20 months.
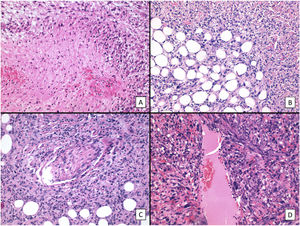
The main histologic findings are shown in Figs. 2–4. Median Breslow depth was 7.2mm and median mitotic count was 18 mitoses per 10 high-power fields. Nineteen tumors (60%) showed ulceration and 22 (73%) had an invasive growth pattern on histology. Necrosis was observed in 10 tumors (33%), lymphovascular invasion in 12 (39%), and perineural invasion in 10 (33%). All the clinicopathologic features and the results of the descriptive statistical analyses are summarized in the supplementary material (Supplementary material 1, Tables 1 and 2, and Supplementary material 2).
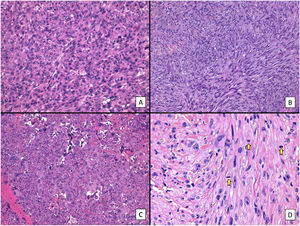
Histologic features of pleomorphic dermal sarcoma (hematoxylin–eosin). A, Dense cell proliferation formed by sheets of epithelioid cells with prominent nucleoli and pronounced pleomorphism and cellular atypia (original magnification ×200). B, Dense cell proliferation formed by bundles of spindle-shaped cells with pronounced pleomorphism and cellular atypia (original magnification ×200). C, Monster cells with large, atypical nuclei, prominent nucleoli, and large cytoplasms with a xanthomatous appearance. Note the numerous multinucleated cells and markedly pyknotic nuclei (original magnification ×200). D, Cell proliferation with marked pleomorphism and atypia, similar to in the previous images, but with multiple mitotic figures (yellow arrows) (original magnification ×400).
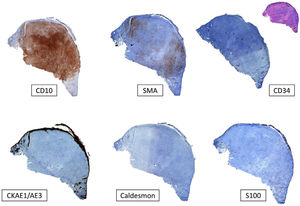
Typical immunohistochemistry panel for pleomorphic dermal sarcoma (PDS). Undifferentiated tumors must always be ruled out before diagnosing PDS. This is done by checking for negative expression of cytokeratins (CKAE1-AE3) for cutaneous squamous cell carcinoma, S100 and/or other melanocytic markers for malignant melanoma, and caldesmon for leiomyosarcoma. CD10 tends to be positive in PDS, although it is a nonspecific marker. There may also be focal positivity for smooth muscle actin (SMA) and vimentin (not shown in the image).
We first conducted a survival analysis for each of the variables using Kaplan–Meier survival curves. Univariate Cox regression analysis was performed for continuous variables. For variables that did not meet the assumption of linearity, iterative loops were used to identify cutoff points for creating groups with significant survival differences. Significance was set at P<0.1. Variables significantly associated with worse disease-free survival were tumor recurrence (P<.001), necrosis (P=.020), lymphovascular invasion (P=.037), perineural invasion (P=.041), and number of mitoses (<18 vs. ≥18) (P=.093) (Fig. 5). Age, tumor size, and tumor thickness (Breslow depth) were not associated with significant differences in survival. Just 1 of the primary tumors (initially diagnosed as PDS) recurred; the other tumors that recurred had been initially diagnosed as AFX. None of the tumors with a mitotic count of less than 10 recurred.
We then performed multivariate Cox regression analysis using the variables with significant differences in the univariate analysis (necrosis, lymphovascular invasion, perineural invasion, and mitotic count). Recurrence was not included in this analysis to avoid losing statistical power (Supplementary material 2). Variables associated with worse survival in the multivariate Cox regression analysis were mitotic count (<18 vs. ≥18) and lymphovascular invasion (P<.05). Notably, individuals with 18 or more mitoses per 10 high-power fields were 5 times as likely to experience recurrence as those with a lower count.
DiscussionDue to its low incidence and the confusion that has historically surrounded its nomenclature, the true aggressive potential and prognosis of PDS are unknown.1,4,6,8,12 Aspects such as clinical behavior, prognosis, and management have been more widely studied in AFX. Most of the information available for PDF is from case reports or small series, and the few studies that have analyzed large groups of patients are mostly descriptive.2–4,13,14 In our series, just 1 of the tumors that recurred was a primary PDS; all other recurrences corresponded to tumors initially diagnosed as AXF. This highlights the need for more thorough histologic examination, as the tumors are morphologically similar and a sufficiently large sample (ideally, a completely excised specimen) is needed to check for features predictive of aggressiveness.14 In a previous study by our group, 75% of PDSs (12/16) had been previously diagnosed as AFX.4 As such, they were probably treated less aggressively and followed less closely, leading to adverse outcomes in many cases.4
Although most studies of PDS to date have been descriptive, there are 2 recent analytical studies of note. In the first, a multicenter study of 92 patients, Persa et al.1 found that an excision margin of less than 2cm and absence of histologic ulceration were risk factors for worse disease-free survival.1 The predictors identified in the second, more recent, study of the Surveillance, Epidemiology, and End Results Program (SEER) database (1911 PDSs) were advanced age, a tumor size of less than 4cm, and metastasis.12 In our study, necrosis, perineural invasion, lymphovascular invasion, and tumor recurrence were identified as prognostic factors for worse disease-free survival in the univariate Cox regression analysis. An association was also observed for mitotic count (≥10 vs. <10). In the multivariate analysis, lymphovascular invasion and a high mitotic count (≥18 vs. <18) were associated with worse disease-free survival, with patients with 18 or more mitoses per 10 high-power fields showing a significantly increased risk (5-fold) of recurrence. Finally, it should be noted that while positive surgical margins during initial excision were not identified as a significant prognostic factor in our statistical analyses, they were an important predictor of local recurrence, as 3 of the 4 patients with metastasis had involved margins and experienced at least 1 local recurrence. These findings are consistent with previous reports.1–3 Margin positivity may not have emerged as a significant prognostic factor in our study due to the bias inherent to the cases from Instituto Valenciano de Oncología (most cases in the series), as many patients had been referred from other hospitals with recurrences rather than primary tumors.
The main prognostic factors that have been identified in soft tissue sarcomas are tumor size and depth, tumor stage, and histologic stage or grade. The most widely used grading system in this setting, that of the Fédération Nationale des Centers de Lutte Contre Le Cancer (FNCLCC), uses 3 independent prognostic factors to grade sarcoma: tumor necrosis, tumor differentiation, and mitotic count (classified as <10, 10–20, or ≥20).15–17 Sarcomas of cutaneous origin, which are more superficial and generally associated with a much better prognosis, are not included in these grading systems. However, logic would suggest that criteria associated with a worse prognosis in deeper tumors with a related lineage may have a similar significance in PDS. Necrosis, lymphovascular invasion, and a higher mitotic count are considered to be independent prognostic factors for aggressiveness in soft tissue sarcomas.15 Based on our findings, they would also appear to be associated with greater aggressiveness (expressed as worse disease-free survival) in PDS. It should also be noted that none of the patients with a mitotic count of less than 10 mitoses per 10 high-power fields experienced recurrence while those with a count of 18 or higher had a 5-fold increased risk of recurrence. These results indicate that a high mitotic count would be the most significant prognostic factor. Perineural invasion has not been identified as a prognostic factor for either cutaneous or soft tissue sarcomas in other studies, although it is a well-known and important marker of aggressiveness in other malignant tumors, the most common and well characterized of which include head and neck squamous cell carcinoma and cutaneous squamous cell carcinoma.18–20 Although this study is one of the largest series of PDS to date (the largest so far, excluding the SEER study, included between 16 and 92 patients), our sample is still relatively small. Accordingly, in the absence of similar findings corroborating our results, we cannot speculate about the true prognostic value of perineural invasion in PDS. However, it must be emphasized that the ability to invade nerve fibers should be considered a trait of aggressiveness in any tumor (regardless of whether or not it is categorized as a prognostic factor). Nerve invasion thus would be expected to suggest more aggressive behavior. In the multivariate analysis, just lymphovascular invasion and a high mitotic count (≥18 vs. <18) retained a significant association with worse disease-free survival. The other significant variables from the univariate analysis (perineural invasion and necrosis) showed a trend toward significance. The absence of significance may be due to the relatively small sample, but if the trend continued in a larger sample, they might achieve significance. Finally, it is notable that all but 1 of the 4 aggressiveness criteria that distinguish PDS from AFX were associated with worse disease-free survival in our series. The feature without significance was subcutaneous invasion, the most common finding in our series (present in all tumors but 1). Subcutaneous invasion is probably the least stringent of these criteria, as there is disagreement on how significant it must be in order to be considered a prognostic factor. (In our study, focal and/or isolated subcutaneous invasion was not included as an exclusive defining factor for PDS.) In addition, many benign or intermediate-grade tumors without aggressive clinical behavior may exhibit subcutaneous invasion.13 Based on our findings, we speculate that subcutaneous invasion probably has less prognostic significance and that the presence of any of the other 3 criteria (necrosis, perineural invasion, and lymphovascular invasion) is truly indicative of greater potential for aggressive behavior and worse disease-free survival.
ConclusionsPDS is an aggressive tumor in which a high mitotic count (≥18 mitoses per 10 high-power fields) and lymphovascular invasion are associated with a higher risk of recurrence and worse disease-free survival. Necrosis and perineural invasion are also probably linked to increased tumor aggressiveness. It is crucial to make an accurate initial diagnosis and avoid misdiagnosing tumors with histologic features of aggressiveness as AFX.
Conflicts of InterestThe authors declare that they have no conflicts of interest.