Folliculotropic mycosis fungoides is a variant that has poor prognosis and a variable clinical presentation. Concerns have been expressed that the current TNMB staging of this tumor may not be useful. A recently developed classification system based on clinical and histologic variables classifies this tumor as early or advanced, a distinction found to correlate with prognosis. The aim of this study was to compare survival in FMF in Colombia between patients with early versus advanced tumors.
Material and methodsRetrospective, observational study of clinical course and outcomes in patients with FMF treated at the National Cancer Institute of Colombia between 2008 and 2020. Survival was compared between early and advanced disease.
ResultsTwenty-one patients (11 with early FMF and 10 with advanced FMF) were studied. Seven patients, all with advanced disease, died. Survival at 5 years was 62% overall and 40% for patients with advanced FMF. No differences were observed when survival was analyzed according to TNMB stage.
ConclusionsTNMB staging is not useful in FMF. The new classification system based on clinicopathologic features appears to provide reliable information for assessing prognosis and guiding treatment decisions.
La micosis fungoide foliculotropa es una variante de mal pronóstico y presentación clínica variada. Se ha planteado que la estadificación TNMB usada para esta neoplasia no es útil. En una propuesta reciente basada en aspectos clínicos e histológicos, se clasifica en enfermedad temprana y avanzada, encontrando diferencias pronósticas entre las dos categorías. El objetivo de este estudio fue comparar la supervivencia de estos dos grupos en nuestra población.
Materiales y métodosSe realizó un estudio observacional retrospectivo de serie de casos donde se evaluó la evolución clínica de los pacientes con micosis fungoide foliculotropa tratados en el Instituto Nacional de Cancerología entre el 2008 y el 2020, realizando un análisis comparativo de supervivencia entre aquellos que tienen enfermedad temprana y enfermedad avanzada.
ResultadosSe incluyó a un total de 21 pacientes, 11 de los cuales presentaban enfermedad temprana y 10 enfermedad avanzada. Se identificaron siete decesos, todos ellos en los pacientes con enfermedad avanzada. La supervivencia global de la población total a cinco años fue del 62%, mientras que para la población con enfermedad avanzada fue del 40%. No hubo diferencias en la supervivencia según la estadificación TNMB.
ConclusiónLa estadificación TNMB no es útil para los pacientes con una micosis fungoide foliculotropa. Por el contrario, la nueva clasificación clínico-patológica parece brindar información pronóstica fiable y permite tomar medidas terapéuticas acordes.
Mycosis fungoides (MF) is the most common primary cutaneous lymphoma, accounting for 50% of cutaneous lymphomas1,2 and 81.8% of the cutaneous lymphomas attended in the Colombian National Cancer Institute (NCI) in 2019,3 with an incidence of 5.7 cases per million population.2,4,5 MF has many clinical, pathological, and histopathological variants, including folliculotropic MF (FMF), which is recognized by the World Health Organization and accounts for 5–10% of MF and 2.8% in our population.2,6–8
FMF is a disease that affects mainly men, with a peak onset in individuals in their 40s and 50s.7,9,10 Its clinical presentation is diverse, characterized initially by follicular involvement and presenting as areas of alopecia, follicular papules, lesions similar to keratosis pilaris, milia, acneiform lesions with cysts and comedones, and leonine facies, although it can also present as classic MF.9,10 FMF is characterized by folliculotropic infiltrate, with predominance of CD4+ cells, with or without epidermotropism and, at times, follicular mucinosis.4,7,9,10
Traditionally, prognosis for FMF is described as that of MF in the tumor stage, with a 5-year survival ranging from 68% to 75%.1,2,11 Response to skin-targeted therapies is typically lower and the prognosis worse than for MF. For this reason, the current Tumor Node Metastasis Blood (TNMB) staging system may not be of use1,10–12 and a different staging has been proposed.2,13 Hodak et al.14 proposed classification of FMF into early-stage or advanced disease. Early-stage disease included patients with patches, alopecia, acneiform lesions similar to keratosis pilaris or milia, and infiltration-free plaques. Advanced disease included patients with clinically infiltrated plaques, which were associated with denser perifollicular infiltrate, a finding in turn associated with worse prognosis. Subsequently, van Santen et al.12 refined this classification with focus on the perifollicular lymphoid infiltrate. Thus, early-stage plaques are characterized by limited perifollicular and intrafollicular infiltrate, with small cells, whereas advanced plaques are characterized by diffuse or confluent infiltrate, comprised of medium or large sized cells. The authors identified differences in survival: 10-year survival was 80% for those with early-stage disease and 25% for those with advanced disease.
In the present study, we describe the clinical and histological characteristics of FMF in a sample of patients, verifying whether FMF was present in patients with early-stage or advanced disease, according to the definition of van Santen et al.,12 through retrospective follow-up of cases attended in the NCI. In addition, a survival analysis was performed according to type of FMF.
MethodsAn observational, retrospective, case–control study was performed in accordance with the STROBE methodological guidelines. Survival, clinical characteristics, and response to treatment of all patients treated in the NCI between January 2008 and June 2020 and with histological diagnosis of FMF based on infiltration of the follicular epithelium by atypical lymphocytes were studied. Patients who had undergone large-cell transformation at the time of the diagnostic biopsy and those with other hematolymphoid neoplasms or solid tumors, and those with incomplete clinical–pathological information were excluded.
Clinical records were the primary information source for the clinical characteristics, treatment, and response outcomes, using SAP® under the ICD-10 code C840 (mycosis fungoides); data were recorded in the RedCap® (Research Electronic Data Capture) platform. After confirmation of diagnosis, the histological slides from the cases selected were assessed by the anatomical pathology department to determine the type of lesion (patch, early-stage plaque, advanced plaque, and tumor) and extent of folliculotropism present in the sample.
The study was conducted according to the tenets of the Declaration of Helsinki and the ethical guidelines for biomedical research prepared by the Council for International Organizations of Medical Sciences (CIOMS) and with parameters established by the national regulations. It was also approved by the institutional review board. Oversight was provided by an independent team of monitors who verified the validity of the information included in RedCAP.
The clinical, histopathological, treatment, and clinical response variables were presented in tables with absolute and relative frequencies in the case of qualitative variables and measures of central tendency and dispersion for quantitative variables. Some variables of interest were broken down using the dynamic tables function in SPSS V19 (IBM Corp., Armonk, NY, USA). Overall survival (OS) and disease-free survival were represented graphically as a function of time to event, estimated using the Kaplan–Meier method. OS was defined as the time in months from the date of diagnosis to the date of last contact and disease-free survival as the time from the date of complete response through the date of local or systemic recurrence. OS was compared in the 2 populations, those with early-stage disease and those with advanced disease. Confirmation of the vital status of each patient was made every 3 months with a maximum observation period of 36 months or until the death of the last patient. The definitions of clinical cutaneous response were based on those published by Olsen et al.15
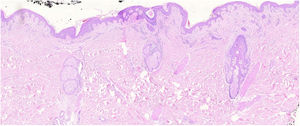
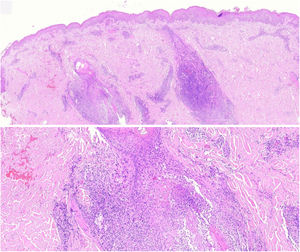
The histopathological slides that gave rise to diagnosis of FMF were evaluated by 2 dermatopathologists (SM and XR), blinded to clinical and follow-up data. The histological parameters assessed were based on the studies by Santen et al.12,16,17 and are as follows: histological subtype (1. early-stage or patch disease; 2. early-stage plaque; 3. advanced plaque; 4. tumor); extension of neoplastic infiltrate (1. scarce; 2. prominently perifollicular and perivascular confined to the perifollicular area; 3. confluent perifollicular and interfollicular; 4. complete diffuse); percentage of atypical cells in infiltrate (<10%; 11–25%; >25%); degree of folliculotropism (mild, moderate, and extensive); epidermotropism (absent or present); syringotropism (absent or present); follicular mucinosis (absent or present); eosinophilic infiltrate (absent or present); Ki-67 expression (less than 10% or greater than 10%); and presence of other microscopic findings such as transformation to large cells or CD8 phenotype. The plaques were assessed with hematoxylin–eosin and with immunohistochemical markers (examples of an early-stage plaque and an advanced one are shown in Fig. 1 and Fig. 2, respectively).
Early-stage plaque. Scarce perifollicular atypical lymphoid infiltrate can be observed, with prominent follicular mucinosis, epidermotropism with linear bands of lymphocytes at the dermal–epidermal junction. Of note is the fibrotic background and poikiloderma, as well as some apoptotic keratinocytes and melanophages.
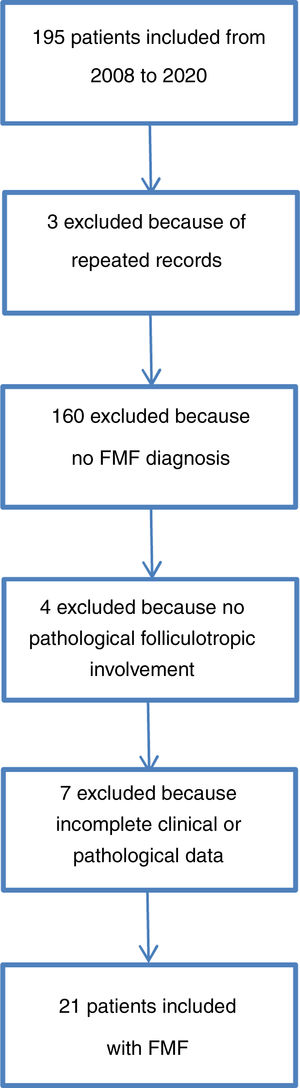
The patient selection process is described in Fig. 3. Between 2008 and 2020, 10.9% of cases of MF attended in the institute were folliculotropic. The clinical and demographic characteristics are summarized in Table 1. The modified severity weight assessment tool (mSWAT) score15 was only recorded in 10 medical records: for those where it was available, the median was 138.5 (12–300). The final study population comprised 21 patients after review of their medical records and assessment of the histopathological slides that confirmed diagnosis of FMF. The pathological features are presented in Table 2. Overall, 95.2% of patients received at least one topical therapy, 66.7% received at least one systemic therapy, and 57.1% received a combination of skin-targeted therapy with systemic therapy. The most frequently used combination was interferon with psoralen plus UV-A (PUVA) therapy (7 patients [33.3%]), followed by combinations that included use of photopheresis with topical steroids in 4 patients (19%).
Clinical characteristics.
| Variable | Outcome |
|---|---|
| Age (mean) | 50.9 years (9.7–85.9) |
| Age at onset of symptoms (mean) | 45.7 years (7–85) |
| Sex, n (%) | |
| Male | 16 (76.2) |
| Female | 5 (23.8) |
| Tumor, n (%) | |
| T1 | 5 (23.9) |
| T2 | 7 (33.3) |
| T3 | 4 (19) |
| T4 | 5 (23.8) |
| Lymph nodes, n (%) | |
| N0 | 19 (90.5) |
| N1 | 0 |
| N2 | 2 (9.5) |
| Metastasis, n (%) | |
| M0 | 21 (100) |
| Blood, n (%) | |
| B0 | 20 (95.2) |
| B1 | 0 |
| B2 | 1 (4.8) |
| Stage, n (%) | |
| IA | 5 (23.8) |
| IB | 7 (33.3) |
| IIB | 4 (19) |
| IIIA | 4 (19) |
| IVB | 1 (4.8) |
| Pruritus, n (%) | |
| No | 4 (19) |
| Yes | 13 (61.9) |
| No data | 314.3) |
| Clinical,an (%) | |
| Macules | 2 (9.5) |
| Patches | 3 (14.3) |
| Plaques | 14 (66.7) |
| Follicular papules | 6 (28.6) |
| Cysts/comedones | 0 (0) |
| Alopecia | 6 (28.6) |
| Tumor | 5 (23.8) |
| Erythroderma | 5 (23.8) |
| Site,an (%) | |
| Head and neck | 15 (71.4) |
| Trunk | 14 (66.7) |
| Upper limbs | 14 (66.7) |
| Lower limbs | 15 (71.4) |
| Buttocks | 9 (42.9) |
Histopathologic features.
| Variable | Outcome |
|---|---|
| Histopathological subtype, n (%) | |
| Early-stage disease | 1 (4.8) |
| Early-stage plaque | 10 (47.6) |
| Advanced plaque | 7 (33.3) |
| Tumor | 3 (14.2) |
| Extent of neoplastic infiltration, n (%) | |
| Scarce | 3 (14.3) |
| Prominently perifollicular and perivascular confined to the perifollicular area | 9 (42.9) |
| Perifollicular and interfollicular confluent | 8 (38.1) |
| Complete diffuse | 1 (4.8) |
| Percentage of atypical cells in the infiltrate, n (%) | |
| Fewer than 10% | 3 (14.3) |
| 11–25% | 6 (28.6) |
| More than 25% | 12 (57.1) |
| Folliculotropism, n (%) | |
| Mild | 3 (14.3) |
| Moderate | 7 (33.3) |
| Extensive | 11 (52.4) |
| Epidermotropism, n (%) | |
| Absent | 8 (38.1) |
| Present | 13 (61.9) |
| Syringotropism, n (%) | |
| Absent | 14 (66.7) |
| Present | 7 (33.3) |
| Follicular mucinosis, n (%) | |
| Absent | 4 (19.0) |
| Present | 17 (81.0) |
| Eosinophilic infiltrate, n (%) | |
| Absent | 14 (66.7) |
| Present | 7 (33.3) |
| Ki-67, n (%) | |
| Less than 10% | 6 (28.6) |
| Greater than 10% | 10 (47.6) |
| No data | 5 (23.8) |
| Other findings, n (%) | |
| Large-cell transformation | 2 (9.5) |
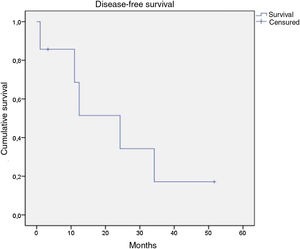
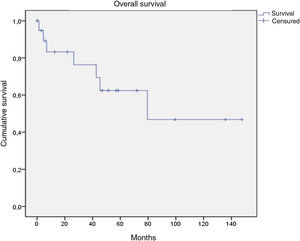
Of the 21 patients in the series, following therapy, 7 reported complete response, 9 partial response, 1 stable disease only, and 2 disease progression from the start of follow-up. Of the 16 patients with complete or partial response, 9 showed disease progression during the study. Of the 7 patients with complete response, 5 relapsed, with a median disease-free survival of 12.4 months (Fig. 4). After a median follow-up of 43.2 months, 7 patients had died, with a median OS of 42.6 months, and a 5-year survival rate of 62% (Fig. 5).
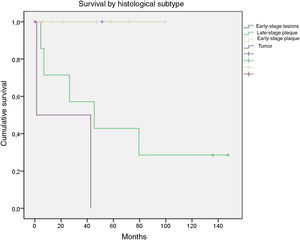
OS was stratified by groups defined as those with early-stage disease, which included patients with early-stage disease or early-stage patches or plaques, and those with advanced disease, which included advanced plaques and tumors. There were no deaths among the 11 patients with early-stage disease; in contrast, of the 10 with advanced disease, only 3 survived until the end of follow-up. On assessing only patients with advanced plaques, 28.6% were alive at the end of follow-up while only 33.3% of those with tumors were alive (Fig. 6).
Contingency tables were drawn up for clinical and pathological variables, with clinical response, disease progression, and OS, as outcomes (Table 3).
Overall response, progression, and death by clinical and histopathological variables.
| Variable | CR | PR | SD | Progressiona | Death |
|---|---|---|---|---|---|
| Sex (n) | |||||
| Female (5) | 40% | 40% | 0% | 60% | 40% |
| Male (16) | 31.3% | 43.8% | 6.3% | 50% | 31.2% |
| Stage (n) | |||||
| IA (5) | 40% | 20% | 20% | 40% | 20% |
| IB (7) | 28.6% | 57.1% | 0% | 57.1% | 75% |
| IIB (4) | 50% | 25% | 0% | 50% | 42.8% |
| IIIA (4) | 25% | 75% | 0% | 50% | 25% |
| IVB (1) | 0% | 0% | 0% | 100% | 100% |
| Subtype (n) | |||||
| Early-stage disease (1) | 0% | 100% | 0% | 0% | 0% |
| Early-stage plaque (10) | 50% | 30% | 10% | 40% | 0% |
| Advanced plaque (7) | 28.6% | 42.9% | 0% | 85.7% | 71.4% |
| Tumor (3) | 0% | 66.6% | 0% | 33.3% | 66.6% |
| Neoplastic infiltrate (n) | |||||
| Scarce (3) | 66.6% | 33.3% | 0% | 33.3% | 0% |
| Perifollicular (9) | 33.3% | 44.4% | 11.1% | 44.4% | 22.2% |
| Perifollicular and interfollicular (8) | 25% | 37.5% | 0% | 62.5% | 50% |
| Diffuse (1) | 0% | 100% | 0% | 100% | 100% |
| Percentage of atypical cells (n) | |||||
| Fewer than 10% (3) | 66.6% | 0% | 33.3% | 33.3% | 0% |
| 11–25% (6) | 33.3% | 50% | 0% | 50% | 0% |
| More than 25% (12) | 25% | 50% | 0% | 58.3% | 58.3% |
| Degree of folilculotropism (n) | |||||
| Mild (3) | 33.3% | 66.6% | 0% | 33.3% | 0% |
| Moderate (7) | 42.9% | 28.6% | 14.3% | 57.1% | 14.3% |
| Extensive (11) | 27.3% | 45.5% | 0% | 54.5% | 54.5% |
| Epidermotropism (n) | |||||
| Absent (8) | 37.5% | 50% | 12.5% | 25% | 37.5% |
| Present (13) | 30.8% | 38.5% | 0% | 69.2% | 30.7% |
| Syringotropism (n) | |||||
| Absent (14) | 21.4% | 50% | 7.1% | 35.7% | 28.5% |
| Present (7) | 57.1% | 28.6% | 0% | 85.7% | 42.8% |
| Follicular mucinosis (n) | |||||
| Absent (4) | 50% | 50% | 0% | 0% | 25% |
| Present (17) | 29.4% | 41.2% | 5.9% | 64.7% | 35.3% |
| Ki-67 (n) | |||||
| Fewer than 10% (6) | 33.3% | 50% | 16.6% | 33.3% | 16.6% |
| More than 10% (10) | 30% | 40% | 0% | 70% | 50% |
| No data (5) | 40% | 40% | 0% | 40% | 20% |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease.
FMF in patients attended in the NCI presented in patients in their fifth decade of life, with a mean age of onset of 50.9 years and a predominance in males (approximate ratio of men to women of 3 to 1), in line with that reported in a review of international literture.9 In that review, the mean age of onset was between 46 and 59 years, with a male to female ratio of between 2 and 5 to 1. Of note is that the mean time elapsed between onset of symptoms and diagnosis in our population was 62.4 months, much larger than the time of 18–48 months in the aforementioned study, perhaps because of prolonged duration of remission, as ours is the only reference center in the country. Another aspect of note is that the increase in the proportion of cases of FMF relative to MF in the institute, 4% in the study from 2011 by Rueda et al.6 compared with 10.9% in our study, is closer to the range of 5–10% reported in the literature.2,7 Although this may be an underestimate due to cases excluded because of incomplete data, we may have seen more cases than those reported in the general population as the NCI is a reference center.
In our study, we found a clear difference in mortality for patients with early-stage disease compared with those with advanced disease, with 5-year OS of 100% in patients with early-stage disease versus 40% for advanced disease. Comparison of mortality by staging showed there was no relationship between the patient's staging and the risk of death (Table 3), supporting the idea that TNMB is not useful in FMF,1,10–12,18 although it is recognized that there were a limited number of patients who could have staging assessed. In addition, mortality was also higher for histological factors, such as neoplastic infiltrate, percentage of atypical cells, degree of folliculotropism, syringotropism, and Ki-67, as the extent of involvement increased.
The results of the present study are similar to the findings of Santen et al.,12 who found differences in the prognosis of patients with plaques, which were classified as early-stage and advanced, based on the density of follicular infiltrate. Patients with early-stage plaques showed a similar behavior to patients with patches and papules, whereas those with advanced-stage plaques behaved in a similar fashion to patients with nodules and tumors, with 5-year OS of 92% for early-stage disease limited to the skin compared with 55% for advanced disease limited to the skin.12 Subsequently, Santen et al.17 confirmed their findings by assessing patients with plaque disease. They found variables associated with poor prognosis were age greater than 60 years, absence of remission after the first treatment, interfollicular epidermotropism, greater than 25% infiltration with atypical cells, expression of Ki-67 greater than 10%, and medium- to large-sized cells, similar to the findings in our study. In contrast, follicular mucinosis was associated with better outcomes in the study by Santen et al. compared with our study, where mortality was higher when mucin was present in the follicular epithelium.
Outcomes were similar to those described by Hodak et al.,14 who classified FMF into early-stage and advanced disease and found a greater perifollicular infiltrate, greater infiltration depth, and greater number of eosinophils and plasmacytes in advanced lesions, as well as a difference in 5-year survival–94% for early-stage disease and 69% for advanced disease. Patients with early-stage disease with disease progression had a greater perifollicular infiltrate. In 2001, Charli-Joseph et al.18 published the validation study of the findings of van Santen et al. in the United States, dividing FMF into early-stage or advanced disease according to the clinical findings and histological involvement in plaques; they obtained a 5-year OS and disease-specific survival (DSS) for the cohort of 89%. The group with early-stage disease had a 5-year OS and DSS of 96%, whereas the corresponding figure in the group with advanced disease was 70%.
In contrast, in Brazil, Deonizio et al.19 found that in their cohort of FMF, there was no difference in survival between early-stage disease (stage IA-IB) and advanced disease (≥stage IIB). Subsequently, Wieser et al.10 reported that the stage with greatest progression was IB and patients with a lower probability of survival were those with stages IVA and IVB, as well as those with large-cell transformation and those aged more than 65 years. Kalay Yildizhan et al.20 also did not find any differences in DSS between those with early-stage disease and those with advanced disease (median of 48 months) or in 5-year or 10-year OS (83% and 69%, respectively), with one of the factors associated with mortality being elevated LDH and large-cell transformation. The above 3 studies classified disease by TNMB stage without taking into account histological findings.
The present study is subject to some limitations. As this was a retrospective study, statistical associations cannot be inferred. In addition, as the patients were attended in a reference center, selection bias may be present, with underestimation of the actual number of patients with FMF, or patients with more severe disease may have been included. Nevertheless, the findings of Hodak et al.14 and van Santen et al.12 are confirmed, validating this approach to prognosis, and also allowing an early aggressive management of the population of patients with advanced plaques.
In conclusion, FMF is an uncommon variant of MF with worse survival than the classic variant, and so the TNMB classification is not useful for estimating prognosis. In our population, the classification that distinguishes between early-stage and advanced disease, based on the clinical and histopathological findings, enabled clear differentiation between 2 groups in terms of outcomes, as has already been demonstrated in other populations. This is likely because it takes into account the presence of a higher tumor load, expressed as the greater atypical lymphoid infiltrate observed histologically but not yet expressed clinically. The greater disease progression and shorter OS observed in patients with a higher number of atypical cells in the infiltrate and with a greater extension of folliculotropism would seem to support this hypothesis. Studies that do not show differences in survival are based on the TNMB staging system and not on differences in histopathological infiltration, thus probably hindering correct identification of patients at greatest risk.
FundingThis article did not receive any funding.