SARS-CoV-2 has caused millions of infections and deaths worldwide and case numbers continue to rise. Besides the effect of the virus on key organs – leading to respiratory illness, anosmia, diarrhea, and fever and other complications – delayed inflammatory reactions to hyaluronic acid dermal fillers, mainly in the face, have also been reported to occur after confirmed SARS-CoV-2 infections and in vaccinated individuals. While delayed inflammatory reactions tend to be self-limiting, they should be diagnosed and treated with corticosteroids, hyaluronidase, and/or antibiotics when necessary. The inflammation is generally not severe, yet these complications are classified as serious adverse events by the US Food and Drug Administration. They appear to be delayed type IV hypersensitivity reactions triggered by the immune system in the presence of SARS-CoV-2 or other viruses, such as those causing influenza, although the underlying mechanisms have not been fully elucidated. Because the longevity of dermal fillers is increasing, while the pandemic continues to evolve and new vaccines are under development, the long-term effects on hyaluronic acid fillers and other bioimplant materials should be studied. Physicians must also be encouraged to report these reactions, however mild, to ensure accurate records.
La pandemia por COVID ha causado hoy en día millones de afectados, continuando su aumento a nivel mundial. Junto con la afectación los órganos diana clave (aparato respiratorio, anosmia, diarrea, fiebre, etc.), se han descrito reacciones inmunológicas tardías en los rellenos dérmicos por ácido hialurónico (AH), fundamentalmente a nivel facial. Estas alteraciones aparecen tanto en pacientes positivos para el virus, independientemente de la sintomatología sistémica, como en pacientes que han recibido vacunación frente al SARS-CoV-2. Aunque las reacciones suelen ser autolimitadas y autoresolutivas, es importante saber diagnosticarlas y en ocasiones establecer tratamiento con corticoides, hialuronidasa y/o antibióticos. Aunque no son graves, la Administración de Alimentos y Medicamentos (FDA) de los Estados Unidos las ha clasificado como evento adverso serio. Los mecanismos que originan estas reacciones no están completamente dilucidados. Parece que son reacciones de hipersensibilidad retardada tipo IV, favorecidas por estímulos inmunológicos que se activan en presencia de la COVID o de otros virus como la gripe. Sin embargo, dado que los rellenos presentan cada vez mayor durabilidad y a que la pandemia continúa su curso, existiendo nuevas vacunas en desarrollo, es esencial la realización de estudios que describan la evolución a largo plazo tanto de los rellenos de AH, como de otros bioimplantes. Así mismo, es esencial alentar a los médicos de que reporten este tipo de reacciones, aunque no revistan gravedad con el objetivo de poder realizar un registro fidedigno de ellas.
Hyaluronic acid (HA) is a glycosaminoglycan that forms part of the extracellular matrix. It is secreted by several types of cells (e.g., fibroblasts, synoviocytes, endothelial cells, smooth muscle cells, adventitial cells, and oocytes1) and is a component of cell and tissue matrix. It stabilizes cellular components, thus regulating osmotic balance, cell proliferation, adhesion, and migration.2
The molecular structure of HA is identical in all organisms and is considered biologically inert, thus minimizing the risk of immunogenicity owing to the absence of protein epitopes.2,3
Its biocompatibility and stability at the injection site, together with its cost-effectiveness, make HA an almost ideal dermal filler.1,2 Furthermore, the expansion of tissue after injection enables HA to activate fibroblasts, thus stimulating the production of collagen.1 The number of nonsurgical cosmetic treatments has continued to rise in Spain despite the pandemic, and more than 35.5% of cases involve HA.4
The discontinuation of bovine HA and improvements in techniques for obtaining the product through biosynthesis of bacteria from streptococcal strains (Streptococcus equi or Streptococcus zooepidemicus) have considerably reduced the frequency of adverse reactions. However, some risk remains owing to the additives used for stabilization and reticulation and the possibility of contamination by proteins or bacterial RNA during the production process.1,5,6
The frequency of adverse reactions ranges from 0.02% to 0.4%,5,7 with percentages of up to 4.25% associated mainly with low-molecular-weight HA.2,3,7,8
Since the first case of pneumonia caused by the new coronavirus SARS-CoV-2 reported in December 2019 in Wuhan, China, the pandemic has led to more than 207780000 infections throughout the world.9,10
There have been reports of delayed inflammatory reactions to HA fillers in patients who have been in contact with SARS-CoV-2 or who developed COVID-1911,12 and of abnormalities affecting dermal fillers after vaccination.13,14 Delayed inflammatory reactions to dermal fillers had been reported before the pandemic and were mainly associated with the common cold or occurred after influenza vaccination.1,2,11,12
Importantly, improvements in the synthesis and production of HA increase the longevity of dermal fillers, with some remaining in place in specific areas of the face for up to 5 years. Therefore, we might expect more frequent reports of COVID-19-induced abnormalities.
Material and MethodsIn this paper, we review the pathophysiology and symptoms of delayed inflammatory reactions to dermal fillers associated with COVID-19. We also analyze the most frequent abnormalities associated with dermal fillers reported after vaccination campaigns and discuss their diagnosis and treatment.
We performed a bibliographic review of the available literature. A search of PubMed using the terms “Fillers AND COVID”, “Dermal Fillers AND COVID”, and “Hyaluronic Acid AND COVID” yielded 77 articles, of which 42 were eventually analyzed. The search was closed on August 28, 2021.
ResultsWe reviewed all case reports and small case series describing abnormalities affecting dermal fillers after SARS-CoV-2 infection.
Munavalli et al.11 were the first authors to describe these abnormalities in several patients. They reported their diagnostic method, treatment, and outcomes and discussed the use of lisinopril as an innovative appropach to the reactions.
Other authors subsequently reported similar cases, and the United States Food and Drug Administration acknowledged the emergence of immunological reactions associated with COVID-19 and vaccination12 and even problems affecting breast implants years after placement.27
DiscussionDelayed inflammatory reactions to HA dermal fillers are classified by time to onset, as follows: early reactions, which occur between 2 weeks and 1 year after injection, and late reactions, which occur more than 1 year after injection. Some authors differentiate between early reactions (first week), intermediate reactions (1 week to 1 month), and late reactions (after more than 1 month). In clinical practice, it is difficult to identify the cause of intermediate and delayed reactions, although their treatment is similar regardless of etiology.7
While the factors leading to the reactions remain unclear, they could be favored by immunologic triggers (e.g., antibodies against HA, residual bacterial RNA or proteins that contaminate HA once produced), bacteria transmitted to the inner surface of the skin during injection and generating biofilms, low-quality products, bacterial infections (e.g., sinusitis, infections resulting from dental procedures), and viral infections (mainly those manifesting with flu-like symptoms or after influenza vaccination).2,7,11,15
Recent improvements in the reticulation and stabilization of HA have led to fillers that are much more resistant to enzymatic degradation, thus increasing their longevity. Given that current fillers last between 2 and 5 years in certain areas of the face, we can expect an increase in the frequency of reactions in the coming years.7,11
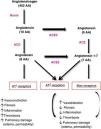
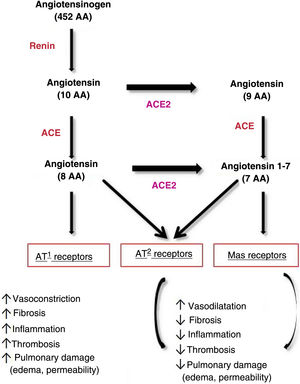
As for pathophysiology, SARS-CoV-2 invades cells through binding of its long membrane protein (S) to the I domain of angiotensin-converting enzyme (ACE) 2. This 1273-amino acid protein projects through the viral envelope, leading to the crown-like appearance. Thus, the virus is anchored to the cell, leading to cellular fusion, release of RNA to the cytoplasm, and infection of the new cell.11,16,17 Some authors have shown that SARS-CoV-2 can only enter cells that express ACE2.17 Importantly, ACE is the target not only of the new coronavirus, but also of several coronaviruses and the influenza virus.18
ACE2 is widely distributed in tissues, although it is particularly abundant in the heart, vessels (vascular endothelium and smooth muscle), intestine, lung (tracheal and bronchial endothelium, type 2 pneumocytes, macrophages), kidneys, testicles, and brain.16 ACE2 levels are also high in the skin, mainly in fibroblasts and keratinocytes, as well as in adipose tissue, which is the most common placement site.11
ACE2 plays a key role in the regulation of arterial blood pressure and antiatherosclerotic mechanisms.18 On binding to ACE2, SARS-CoV-2 modifies its physiological activity by altering the balance between ACE/ACE2 and angiotensin II/angiotensin,1–7 leading to an increase in angiotensin II and its binding to the AT1 receptor and favoring deleterious effects, such as vasoconstriction, fibrosis, inflammation, thrombosis, lung damage with edema, and increased permeability16,17 (Fig. 1).
Pathways of angiotensin metabolism16. ACE indicates angiotensin-converting enzyme; AT, angiotensin.
Adapted from Verdecchia et al. Eur J Intern Med. 2020.
In addition, COVID-19 increases expression of inflammatory mediators (mainly interleukin [IL] 6, IL-2, IL-8, monocyte chemoattractant protein 1, interferon γ inducible protein 10) and induces recruitment of inflammatory cells, together with a deficient interferon response and production of antibodies.17
The hypersensitivity reactions that give way to delayed inflammatory reactions are thought to be type IV, triggered by T lymphocytes and mediated by CD4. These may be induced by viral infections, which in turn activate macrophages.13 Seasonal prevalence has been reported, with reactions occurring mainly in fall and winter or after influenza infection.2
The degree of activation of the immune system also depends on the molecular weight of HA. High-molecular-weight HA mainly exerts an anti-inflammatory effect, whereas low-molecular-weight HA (<500kDa) is proinflammatory and may act as an endogenous signal, activating the immune system itself.2,3 Furthermore, the lower concentration and viscosity increase immunogenicity, because the increased rate of metabolism and degradation increases the area of low-molecular-weight HA exposed and of fragments of HA with a proinflammatory effect.3 Thus, dermal fillers lead to low-molecular-weight HA owing to degradation of the product and to binding agents.2
HA acts as the main ligand of CD44, a glycoprotein expressed in mammalian cells that intervenes in cell signaling processes and is increased in viral infections. Its binding to HA leads to activation and recruitment of lymphocytes.2
Some authors have also suggested that systemic inflammatory responses, such as those caused by flu-like illness, can favor degradation of dermal fillers owing to the production of free radicals, thus giving way to fragments of low-molecular-weight HA that act as CD44 ligands. Furthermore, the increase in low-molecular-weight HA leads to recruitment of lymphocytes to the area of the filler where the HA concentration is strongest.
Therefore, while the sensitization process is not completely clear,3 the presence of filler itself seems to be a risk factor for hypersensitivity reactions in the presence of systemic infection, and this in turn seems to be the cause of edema affecting HA fillers after infection by SARS-CoV-2.2
Modification of HA molecules to increase the longevity of the fillers entails a greater presence of impurities, which are associated with an increase in hypersensitivity reactions.13 Similar findings have been reported for HA when used in other medical techniques.19
The formulation of granulomas and fibrosis may be due to the absence of total phagocytosis of the HA fragments, combined with the formation of biofilm and activation of T cells that are common after implantation of biomaterials. Increased expression of ACE2 in the skin plays a key role in maintaining an appropriate immune response. Alterations in free ACE produced by the virus increase the amount of angiotensin II and the probability of a proinflammatory response that can also affect the dermis.19
As mentioned above, ACE2 levels are high in fibroblasts and keratinocytes. The enzyme is especially abundant in the endothelium and mid and deep dermis and in the subcutaneous microvasculature. Given that HA stimulates angiogenesis, ACE2 levels may increase at the sites of short- or long-term implants, thus increasing the likelihood of delayed reactions after acute infection by SARS-CoV-2 or vaccination.11
COVID-19 also leads to complement activation, especially in subcutaneous fatty tissue, leading to microvasculature abnormalities, endothelial cell denudation, basement membrane zone reduplication, and small thrombi.20
Individual characteristics can also play a role in the development of a delayed inflammatory reaction. The combination of the haplotypes HLA-B*08 and DR1*03 increased 4-fold the likelihood of late-onset adverse events after injection of dermal filler.5,19,21
Delayed inflammatory reactions usually manifest as edema, abnormal skin coloration (mainly erythema), tissue induration, and potentially painful nodules.7,15,22
According to a study by the International Society for Dermatology and Aesthetic Surgery, 15% of patients experienced swelling after their filler injections.13 Munavalli et al.11 were the first to report facial filler abnormalities in patients who had contact with COVID or were vaccinated in January 2021. Patients experienced burning sensation or swelling at the site where the filler was injected, in addition to facial edema, erythema, and increased sensitivity. Some patients reported a transient improvement lasting a few days, albeit with subsequent increased reddening and indurated nodules that occasionally progressed to intermittent erythema. While the patients progressed favorably, they did require corticosteroids, antibiotics, hyaluronidase, and, in 1 case, lisinopril.
The start of the vaccination campaign was followed by reports of inflammatory reactions associated with Pfizer BioNTech (BNT162b2) and Moderna (mRNA-1273).14,23 Data on cases recorded in clinical trials show 414 reactions in persons vaccinated from December 2020 to February 2021.14 Symptoms appeared 12–24hours after vaccination. The reactions comprised mainly vesicular, urticarial, macular, and papular eruptions, as well as swelling at the site of the facial filler (9 cases), pernio/chilblains (8 cases), varicella zoster (10 cases), herpes simplex (4 cases), and nonspecific rashes in the infants of vaccinated breastfeeding mothers (4 cases). The reactions were generally mild.14
The authors attributed the reactions to T lymphocyte–mediated delayed hypersensitivity, associating them with excipients such as neomycin, thiomersal, and polyethylene glycol. Therefore, they were not considered contraindications to further doses of the vaccine.14 Another proposed cause was stimulation of hypersensitivity by an immunologic trigger, as previously reported after viral disease and influenza vaccine.14,23 The authors count as limitations to their study incomplete follow-up and information bias owing to the tendency to report mainly severe reactions.14
The US Food and Drug Administration recognized facial swelling as an adverse effect in patients who had previously received dermal fillers. Edema was considered a severe effect, even though the 3 cases reported during phase 3 of the Moderna trial were successfully treated with corticosteroids and antihistamines.12,23 The US Food and Drug Administration stated that filler abnormalities could be caused by the inflammatory response arising from the interaction between the immune response caused by vaccination and the dermal filler.12 The registry did not identify subsequent complications or sequelae, and patients responded appropriately to topical corticosteroids, antihistamines, and analgesics. Antibiotics were required on occasions where the patient was thought to be developing cellulitis.
Although treatment was always administered on an individual basis, most patients initially received methylprednisolone 1mg/kg for 8–14 days, antibiotics for 1–3 weeks (mainly doxicillin, although trimethoprim–sulfamethoxazole was also administered), and hyaluronidase, with occasional repeated doses in the case of increased swelling after the first injection. Moreover, poorer responses required the addition of 5-fluorouracil, long-term corticosteroids, and antihistamines,11,12 although these are of limited value.8 Oral corticosteroids are the initial therapy for delayed inflammatory reactions after vaccination.19
Importantly, a skin test with 0.1mL of hyaluronidase should be carried out before treatment.8
Recurrence of inflammation is common, and patients should be warned about this possibility.8 Some patients can experience a delayed inflammatory reaction to fillers owing to COVID-19, even if they do not present symptoms of the disease.22
Low-dose oral lisinopril (5mg) has been reported to be successful, with resolution of edema at 24–48hours.11,24,25 Lisinopril can reduce delayed inflammatory reactions caused by vaccination and has been proposed as premedication before vaccination in patients with a history of delayed inflammatory reaction to HA filler or who have experienced reactions after the first vaccination.19 Its benefit arises from its ability to block ACE1 production. This reduces production of angiotensin II and the substate for ACE2, thus offsetting the action of COVID-19. Furthermore, given its rapid onset of action, lisinopril diminishes secretion of aldosterone by the adrenal cortex, thus increasing excretion of sodium and, therefore, water outflow. This leads to rapid resolution of the facial edema associated with delayed inflammatory reaction.25 However, no studies have yet demonstrated its benefits.19,25
Naouri et al.22 reported cases of granuloma formation in patients who received interferon (cytokines). In this setting, granuloma may be caused by the cytokine storm resulting from SARS-CoV-2 infection. Before the pandemic, immunologic complications of fillers were reported in 0.8%–0.9% of cases.22
Delayed inflammatory reactions associated with SARS-CoV-2 generally self-resolve in days or weeks.8,22 In the case of painless nodules measuring less than 0.5cm, patients are recommended to be vigilant. In the case of larger nodules with edema and erythema that are painful and do not improve, treatment should be initiated. The nodules are usually inflammatory in nature, although the possibility that they are due to an infectious process caused by biofilms and atypical microorganisms must be ruled out. Culture for aerobic and anaerobic bacteria, mycobacteria, and fungi is necessary if the nodule fluctuates in size.8
Ghasemi et al.12 reported several similar cases. One patient developed symptoms following the injection of filler 2 days after receiving the Moderna vaccine. In the case of patients with a COVID-positive polymerase chain reaction assay result, symptoms developed after 2 weeks. The nodules resolved 6 weeks later.11
In June 2021, Michon8 also reported 2 cases of delayed inflammatory reaction after administration of the Pfizer vaccine. In the first case, the patient experienced reddening and swelling affecting the tear trough, where an HA filler had been injected 6 weeks earlier with no complications. The patient also developed flu-like symptoms, such as tiredness, headache, joint pain, and anorexia, which resolved in the following days. The adverse reaction itself had resolved spontaneously by day 5. In the second case, the patient received the first dose of the Pfizer vaccine 9 months after receiving panfacial injections of filler. Some days after vaccination, she developed intermittent facial swelling and reddening that mainly affected the cheeks and area under the eye. This lasted approximately 1 day. Three weeks later, she had developed undereye swelling that was worse than before, although no erythema or nodules were present. She was treated with hyaluronidase, and her condition resolved completely.
While not the object of the present review, it is worth mentioning changes in breast implants following injection of the Pfizer, AstraZeneca, and Janssen vaccines.26 Most cases progressed perfectly with analgesic and anti-inflammatory treatment, although in 1 case, the patient developed an infection that required surgical lavage and withdrawal of the prosthesis.26 Restifo27 reported the case of capsular contracture of a breast implant 20 days after administration of the second dose of the Pfizer vaccine.
The duration of delayed inflammatory reactions following COVID-19 seems to be associated with the amount of the initial filler, the technique (bolus/nonbolus), and the duration of the placement.25 Moreover, several inflammatory cytokines resulting from COVID-19 are potent inducers of the metabolism of HA.28 Altered synthesis and deposition of HA is well established in respiratory distress syndrome.28 Depolymerization of HA in airway epithelial cells triggers the inflammatory cascade that leads to production of cytokines.29 In addition, proinflammatory cytokines stimulate exudation of HA in the airway itself.29 Consequently, SARS-CoV-2 clearly interacts with HA, thereby modifying it.
Guidelines on dermal fillers were published in March 2020, although the pandemic has, to a certain extent, necessitated modifications. Strict asepsis and prophylaxis to cover the risk of infection are key to ensuring safety during treatment. While the pandemic continues, the ideal approach would undoubtedly be serology testing to determine the patient's immune status.21
Goodman et al.21 proposed a model for management in the cosmetic medicine clinic during the pandemic. The model comprises a series of points: (a) administration of a health questionnaire for screening purposes and to determine risk contacts; (b) use of telemedicine when possible; (c) identification of patients at high risk of developing severe COVID-19 (smokers, diabetics, obese persons, elderly persons, persons with comorbid conditions) and deferring cosmetic treatment until the risk has decreased; (d) avoiding combination treatment, since this takes longer and increases risk, with the recommendation to stage treatments; (e) maintaining social distancing and regular disinfection in the waiting room; (f) noncontact temperature testing; (g) stressing the importance of hand washing on the days following treatment, since patients frequently touch the areas of the face where the filler was injected; and (h) appropriate use of personal protective equipment; (i) in the case of a high risk of transmission, it is necessary to consider deferring treatment until the risk decreases.
In addition to these principles, the authors stress that it is always necessary to have hyaluronidase ready in case the patient presents signs of vascular occlusion. The skin should be disinfected, preferably with alcohol or povidone iodine. As little pressure as possible should be applied during the injection, which should be performed slowly, with subsequent massage of the area to ensure that the filler is evenly distributed.21
An interval of at least 3 weeks after COVID-19 vaccination should be left before injecting filler in order to avoid the peak immune response, which occurs at 21 days.8
In conclusion, the true etiology of delayed immunologic reactions appearing in association with HA fillers and SARS-CoV-2 infection or with antibodies generated after vaccination remains unclear. Since delayed immunologic reactions secondary to vaccination are mild and self-limiting, the possibility of complications arising from facial filler should not discourage vaccination. An interval of at least 3 weeks should be left between vaccination against COVID-19 and injection of a filler in order to avoid the peak immune response at 21 days. Fillers should be avoided in patients with the combination of HLA B*08 and DRB1*03. Interviews with several cosmetic physicians revealed a certain degree of concern over the increased frequency of delayed inflammatory reactions, which were not observed in the pre-COVID era. The respondents’ subjective perception is that these are due to vaccination or to the disease itself. Given that these reactions do not require treatment or can be managed with low-dose corticosteroids, most cases go unreported, with the result that there are no reliable records. We highlight the importance of appropriate patient selection and follow-up and of ensuring that the cosmetic procedures are carried out by appropriately trained professionals who can diagnose and treat potential complications. Patients should be aware of the risks of undergoing these treatments with unqualified professionals.
FundingThe authors declare that no funding was received for the present study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.