Allergic contact dermatitis induced by the use of ophthalmic topical drugs is one of the most common causes of eyelid dermatitis. The introduction of new formulations, both of active ingredients and excipients, and the lack of marketing in some of them, makes patch testing in patients whose source of contact are topical ophthalmic drugs truly challenging. Across this manuscript, most, if not all, topical ophthalmic drugs used in our national health system have been collected, including information on the allergens available, and the concentration and vehicle advised for those that still remain unavailable.
La dermatitis alérgica de contacto inducida por el uso de productos oftálmicos es una de las causas más frecuentes de dermatitis palpebral. La introducción de nuevas formulaciones, tanto de principios activos como de excipientes, así como la ausencia de comercialización de algunos de ellos, se convierte en un verdadero reto el estudio de pruebas epicutáneas en pacientes cuya fuente de contacto son los productos oftálmicos. A lo largo del manuscrito se han recogido los productos de uso oftálmico más relevantes en nuestro Sistema Nacional de Salud, incluyendo la información referente a los alérgenos comercializados, así como la concentración y vehículo recomendado para aquellos que no se encuentran disponibles comercialmente.
Allergic contact dermatitis (ACD) of the eyelid and/or periocular area is one of the most common causes of eczematous eruptions in this region,1,2 followed by atopic dermatitis, seborrheic dermatitis, and psoriasis. There appears to be a predilection for the female sex.1 Undoubtedly, ACD can be a diagnostic challenge if a targeted and comprehensive medical history is not conducted, including the patient's personal and family history of interest, the current or past presence of clinical signs of another dermatosis, and the possible chronological relationship between the application of ophthalmic products (both prescription and over-the-counter drugs). Although we do not have specific epidemiological data of ACD after the use ophthalmic products, recent reports have confirmed an increase in the number of cases reported in recent years, both due to known allergens, such as benzalkonium chloride, and new molecules as well.
There are differences in the prescription of active ingredients according to the country, suggesting different sensitization rates for marketed molecules, which is why we through it was more interesting for our field to review the ophthalmic products prescribed nationwide.
Undoubtedly, the biggest challenge in studying these patients is to put together a complete battery of allergens that allows for proper patch testing. Although the trend is to use unmodified marketed products (without separating their different components - active ingredient(s), excipients), the truth is is that the interpretation of reactions must be done with caution since this methodology does not conclude which allergen is responsible for sensitization. Hence the importance of having complete series of allergens fully available, or collaboration from the manufacturer.
The objective of this article is to review the most relevant literature on ophthalmic product-induced eyelid ACD, providing the main allergens and vehicles based on the type of pharmacological class to study them through patch testing. Therefore, a bibliographic search was conducted including articles considered most relevant (case series or more comprehensive or updated literature reviews, and reports of interest on commonly used drugs not included in the reviews) published in the past 10 years in the PubMed database as of May 2023. The keyterms used in the search section were (eyelid contact dermatitis) / (palpebral dermatitis) / (periocular dermatitis) / (periorbital dermatitis) AND (ophthalmic medications) / (ophthalmic products) / (eye products) / (eyedrops). Information on the composition of marketed ophthalmic products and their use was obtained by entering the trade names of the corresponding drugs into the Spanish Agency of Medicines and Medical Devices (CIMA) official website. The sections of this article are categorized based on the therapeutic classes by 3 independent reviewers, who manually selected and reviewed the articles, drafted the text, and eventually conducted a final joint review.
Clinical presentationLesions are mainly located on the eyelid and/or periocular area, usually unilateral (except when treatment is applied to both eyes), and primarily in the form of dermatitis (in its different phases: acute, subacute, and chronic). Itching is usually the main symptom. Some patients also describe a burning or foreign body sensation.2 Conjunctivitis can be a clinical form of ophthalmic ACD, although suspicious sensitization component will likely be evaluated by an ophthalmologist, and it will be considered essential when considering referral to dermatology for patch testing.
The clinical presentation of dermatitis can be identified through different patterns already described in the literature,3 where the drip pattern seems to be the most characteristic of all. Erythematous-scaly plaques are usually much more frequent than the presence of vesiculation. In case of vesiculation, a possible herpes simplex virus infection should be ruled out. Differential diagnoses can include diseases such as rosacea, dermatomyositis, or preseptal cellulitis.3 The self-transported (hand-face), airborne,4 and connubial forms5 of dermatitis should be considered in these patients, whose contact source of the causative allergen is usually not of ophthalmological origin. The absence of lesion improvement despite proper treatment with anti-inflammatories (topical corticosteroids and/or calcineurin inhibitors) should raise suspicion of corticosteroid sensitization,6 or excipients sensitization such as propylene glycol.7
Patch testingIn addition to the Spanish standard series, the Spanish Working Group of Research in Contact Dermatitis and Cutaneous Allergy (GEIDAC)8 recommends adding the allergens included in the problematic product(s) (eye drops, solutions, etc.). Most active ingredients are not marketed, therefore, in general, the “unmodified” product per se is often used.
Classic preservatives such as benzalkonium chloride, propylene glycol, EDTA, or thimerosal, among others, are available in marketed preparations, although they are not all are present in different ophthalmic preparations.
Late readings past 96hours (preferably at 7 and 14 days) are recommended since corticosteroids or aminoglycosides can show positive reactions at a later point in time.9 The Repeat Open Application Test (ROAT) with eye drops on the antecubital flexure twice a day for 2 to 3 weeks may be very useful, if neccesary. ROAT10 can be performed before patch testing to confirm suspicion of ACD to it, or after patch testing, when, although they still test negative for the product, suspicion of sensitization remains high. The use test (applying the ophthalmic product as instructed) may be useful when both the ROAT and patch testing test negative,11 even in cases of conjunctivitis.12 The prick test can be interesting to study latex hypersensitivity as the causative agent for eyelid ACD.13 The scratch test may be useful in selected cases.14 Collaboration from the manufacturer when requesting the product composition on an individual basis is usually scarce, which complicates patient study even more. The ophthalmic series commercialized by MartiTor includes a total of 20 allergens (Table 1),15 and may be useful in some cases, although some active ingredients and/or excipients are still missing, meaning that a complete ophthalmology series cannot be created.
Series of ophthalmic medication marketed by Martitor.
| Atropine sulfate 1% water |
| Benzalkonium chloride 0.1% water |
| Chloramphenicol 5% vaseline |
| Chlorhexidine digluconate 0.5% water |
| Chlorhexidine digluconate 1% water |
| Chlorotetracycline HCl 1% vaseline |
| Dexpanthenol 5% vaseline |
| Diclofenac 5% vaseline |
| Disodium edetate (EDTA) 1% vaseline |
| Fusidic acid 2% vaseline |
| Gentamicin sulfate 20% vaseline |
| Kanamycin sulfate 10% vaseline |
| Neomycin sulfate 20% vaseline |
| Oxytetracycline 3% vaseline |
| Phenylephrine hydrochloride 10% water |
| Pilocarpine hydrochloride 1% water |
| Polymyxin B sulfate 3% vaseline |
| Prednisolone 1% vaseline |
| Sodium disulfite 1% vaseline |
| Tetracaine HCl 1% vaseline |
| Thiomersal 0.1% vaseline |
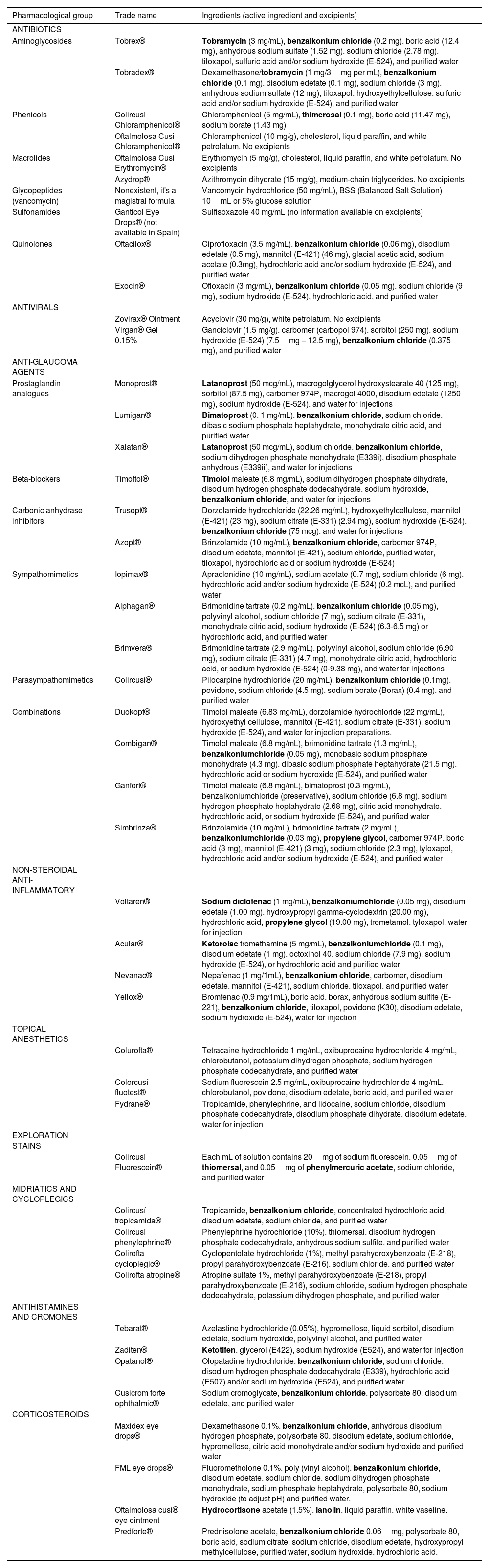
Since the list of marketed ophthalmic products is extensive, information has focused on eye drops and ophthalmic ointments alone. Table 2 lists the different trade names with their active ingredients and excipients.
Main prescription ophthalmic products, pharmacological group, trade name, active ingredient, and excipients.
| Pharmacological group | Trade name | Ingredients (active ingredient and excipients) |
|---|---|---|
| ANTIBIOTICS | ||
| Aminoglycosides | Tobrex® | Tobramycin (3 mg/mL), benzalkonium chloride (0.2 mg), boric acid (12.4 mg), anhydrous sodium sulfate (1.52 mg), sodium chloride (2.78 mg), tiloxapol, sulfuric acid and/or sodium hydroxide (E-524), and purified water |
| Tobradex® | Dexamethasone/tobramycin (1 mg/3mg per mL), benzalkonium chloride (0.1 mg), disodium edetate (0.1 mg), sodium chloride (3 mg), anhydrous sodium sulfate (12 mg), tiloxapol, hydroxyethylcellulose, sulfuric acid and/or sodium hydroxide (E-524), and purified water | |
| Phenicols | Colircusí Chloramphenicol® | Chloramphenicol (5 mg/mL), thimerosal (0.1 mg), boric acid (11.47 mg), sodium borate (1.43 mg) |
| Oftalmolosa Cusi Chloramphenicol® | Chloramphenicol (10 mg/g), cholesterol, liquid paraffin, and white petrolatum. No excipients | |
| Macrolides | Oftalmolosa Cusi Erythromycin® | Erythromycin (5 mg/g), cholesterol, liquid paraffin, and white petrolatum. No excipients |
| Azydrop® | Azithromycin dihydrate (15 mg/g), medium-chain triglycerides. No excipients | |
| Glycopeptides (vancomycin) | Nonexistent, it's a magistral formula | Vancomycin hydrochloride (50 mg/mL), BSS (Balanced Salt Solution) 10mL or 5% glucose solution |
| Sulfonamides | Ganticol Eye Drops® (not available in Spain) | Sulfisoxazole 40 mg/mL (no information available on excipients) |
| Quinolones | Oftacilox® | Ciprofloxacin (3.5 mg/mL), benzalkonium chloride (0.06 mg), disodium edetate (0.5 mg), mannitol (E-421) (46 mg), glacial acetic acid, sodium acetate (0.3mg), hydrochloric acid and/or sodium hydroxide (E-524), and purified water |
| Exocin® | Ofloxacin (3 mg/mL), benzalkonium chloride (0.05 mg), sodium chloride (9 mg), sodium hydroxide (E-524), hydrochloric acid, and purified water | |
| ANTIVIRALS | ||
| Zovirax® Ointment | Acyclovir (30 mg/g), white petrolatum. No excipients | |
| Virgan® Gel 0.15% | Ganciclovir (1.5 mg/g), carbomer (carbopol 974), sorbitol (250 mg), sodium hydroxide (E-524) (7.5mg – 12.5 mg), benzalkonium chloride (0.375 mg), and purified water | |
| ANTI-GLAUCOMA AGENTS | ||
| Prostaglandin analogues | Monoprost® | Latanoprost (50 mcg/mL), macrogolglycerol hydroxystearate 40 (125 mg), sorbitol (87.5 mg), carbomer 974P, macrogol 4000, disodium edetate (1250 mg), sodium hydroxide (E-524), and water for injections |
| Lumigan® | Bimatoprost (0. 1 mg/mL), benzalkonium chloride, sodium chloride, dibasic sodium phosphate heptahydrate, monohydrate citric acid, and purified water | |
| Xalatan® | Latanoprost (50 mcg/mL), sodium chloride, benzalkonium chloride, sodium dihydrogen phosphate monohydrate (E339i), disodium phosphate anhydrous (E339ii), and water for injections | |
| Beta-blockers | Timoftol® | Timolol maleate (6.8 mg/mL), sodium dihydrogen phosphate dihydrate, disodium hydrogen phosphate dodecahydrate, sodium hydroxide, benzalkonium chloride, and water for injections |
| Carbonic anhydrase inhibitors | Trusopt® | Dorzolamide hydrochloride (22.26 mg/mL), hydroxyethylcellulose, mannitol (E-421) (23 mg), sodium citrate (E-331) (2.94 mg), sodium hydroxide (E-524), benzalkonium chloride (75 mcg), and water for injections |
| Azopt® | Brinzolamide (10 mg/mL), benzalkonium chloride, carbomer 974P, disodium edetate, mannitol (E-421), sodium chloride, purified water, tiloxapol, hydrochloric acid or sodium hydroxide (E-524) | |
| Sympathomimetics | Iopimax® | Apraclonidine (10 mg/mL), sodium acetate (0.7 mg), sodium chloride (6 mg), hydrochloric acid and/or sodium hydroxide (E-524) (0.2 mcL), and purified water |
| Alphagan® | Brimonidine tartrate (0.2 mg/mL), benzalkonium chloride (0.05 mg), polyvinyl alcohol, sodium chloride (7 mg), sodium citrate (E-331), monohydrate citric acid, sodium hydroxide (E-524) (6.3-6.5 mg) or hydrochloric acid, and purified water | |
| Brimvera® | Brimonidine tartrate (2.9 mg/mL), polyvinyl alcohol, sodium chloride (6.90 mg), sodium citrate (E-331) (4.7 mg), monohydrate citric acid, hydrochloric acid, or sodium hydroxide (E-524) (0-9.38 mg), and water for injections | |
| Parasympathomimetics | Colircusi® | Pilocarpine hydrochloride (20 mg/mL), benzalkonium chloride (0.1mg), povidone, sodium chloride (4.5 mg), sodium borate (Borax) (0.4 mg), and purified water |
| Combinations | Duokopt® | Timolol maleate (6.83 mg/mL), dorzolamide hydrochloride (22 mg/mL), hydroxyethyl cellulose, mannitol (E-421), sodium citrate (E-331), sodium hydroxide (E-524), and water for injection preparations. |
| Combigan® | Timolol maleate (6.8 mg/mL), brimonidine tartrate (1.3 mg/mL), benzalkoniumchloride (0.05 mg), monobasic sodium phosphate monohydrate (4.3 mg), dibasic sodium phosphate heptahydrate (21.5 mg), hydrochloric acid or sodium hydroxide (E-524), and purified water | |
| Ganfort® | Timolol maleate (6.8 mg/mL), bimatoprost (0.3 mg/mL), benzalkoniumchloride (preservative), sodium chloride (6.8 mg), sodium hydrogen phosphate heptahydrate (2.68 mg), citric acid monohydrate, hydrochloric acid, or sodium hydroxide (E-524), and purified water | |
| Simbrinza® | Brinzolamide (10 mg/mL), brimonidine tartrate (2 mg/mL), benzalkoniumchloride (0.03 mg), propylene glycol, carbomer 974P, boric acid (3 mg), mannitol (E-421) (3 mg), sodium chloride (2.3 mg), tyloxapol, hydrochloric acid and/or sodium hydroxide (E-524), and purified water | |
| NON-STEROIDAL ANTI-INFLAMMATORY | ||
| Voltaren® | Sodium diclofenac (1 mg/mL), benzalkoniumchloride (0.05 mg), disodium edetate (1.00 mg), hydroxypropyl gamma-cyclodextrin (20.00 mg), hydrochloric acid, propylene glycol (19.00 mg), trometamol, tyloxapol, water for injection | |
| Acular® | Ketorolac tromethamine (5 mg/mL), benzalkoniumchloride (0.1 mg), disodium edetate (1 mg), octoxinol 40, sodium chloride (7.9 mg), sodium hydroxide (E-524), or hydrochloric acid and purified water | |
| Nevanac® | Nepafenac (1 mg/1mL), benzalkonium chloride, carbomer, disodium edetate, mannitol (E-421), sodium chloride, tiloxapol, and purified water | |
| Yellox® | Bromfenac (0.9 mg/1mL), boric acid, borax, anhydrous sodium sulfite (E-221), benzalkonium chloride, tiloxapol, povidone (K30), disodium edetate, sodium hydroxide (E-524), water for injection | |
| TOPICAL ANESTHETICS | ||
| Colurofta® | Tetracaine hydrochloride 1 mg/mL, oxibuprocaine hydrochloride 4 mg/mL, chlorobutanol, potassium dihydrogen phosphate, sodium hydrogen phosphate dodecahydrate, and purified water | |
| Colorcusí fluotest® | Sodium fluorescein 2.5 mg/mL, oxibuprocaine hydrochloride 4 mg/mL, chlorobutanol, povidone, disodium edetate, boric acid, and purified water | |
| Fydrane® | Tropicamide, phenylephrine, and lidocaine, sodium chloride, disodium phosphate dodecahydrate, disodium phosphate dihydrate, disodium edetate, water for injection | |
| EXPLORATION STAINS | ||
| Colircusí Fluorescein® | Each mL of solution contains 20mg of sodium fluorescein, 0.05mg of thiomersal, and 0.05mg of phenylmercuric acetate, sodium chloride, and purified water | |
| MIDRIATICS AND CYCLOPLEGICS | ||
| Colircusí tropicamida® | Tropicamide, benzalkonium chloride, concentrated hydrochloric acid, disodium edetate, sodium chloride, and purified water | |
| Colircusí phenylephrine® | Phenylephrine hydrochloride (10%), thiomersal, disodium hydrogen phosphate dodecahydrate, anhydrous sodium sulfite, and purified water | |
| Colirofta cycloplegic® | Cyclopentolate hydrochloride (1%), methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), sodium chloride, and purified water | |
| Colirofta atropine® | Atropine sulfate 1%, methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), sodium chloride, sodium hydrogen phosphate dodecahydrate, potassium dihydrogen phosphate, and purified water | |
| ANTIHISTAMINES AND CROMONES | ||
| Tebarat® | Azelastine hydrochloride (0.05%), hypromellose, liquid sorbitol, disodium edetate, sodium hydroxide, polyvinyl alcohol, and purified water | |
| Zaditen® | Ketotifen, glycerol (E422), sodium hydroxide (E524), and water for injection | |
| Opatanol® | Olopatadine hydrochloride, benzalkonium chloride, sodium chloride, disodium hydrogen phosphate dodecahydrate (E339), hydrochloric acid (E507) and/or sodium hydroxide (E524), and purified water | |
| Cusicrom forte ophthalmic® | Sodium cromoglycate, benzalkonium chloride, polysorbate 80, disodium edetate, and purified water | |
| CORTICOSTEROIDS | ||
| Maxidex eye drops® | Dexamethasone 0.1%, benzalkonium chloride, anhydrous disodium hydrogen phosphate, polysorbate 80, disodium edetate, sodium chloride, hypromellose, citric acid monohydrate and/or sodium hydroxide and purified water | |
| FML eye drops® | Fluorometholone 0.1%, poly (vinyl alcohol), benzalkonium chloride, disodium edetate, sodium chloride, sodium dihydrogen phosphate monohydrate, sodium phosphate heptahydrate, polysorbate 80, sodium hydroxide (to adjust pH) and purified water. | |
| Oftalmolosa cusi® eye ointment | Hydrocortisone acetate (1.5%), lanolin, liquid paraffin, white vaseline. | |
| Predforte® | Prednisolone acetate, benzalkonium chloride 0.06mg, polysorbate 80, boric acid, sodium citrate, sodium chloride, disodium edetate, hydroxypropyl methylcellulose, purified water, sodium hydroxide, hydrochloric acid. |
The most frequently published allergens in the literature have been highlighted in bold.
They are used to treat bacterial conjunctivitis and as preoperative prophylaxis. Topical application has been involved in allergic reactions ranging from mild to life-threatening. Aminoglycosides, sulfonamides, and polypeptides are among the most allergenic classes of topically applied antibiotics.16 No reports have ever been published in the scientific medical literature on allergic contact dermatitis to topical ophthalmic formulations of tetracyclines and fluoroquinolones.17
AminoglycosidesNeomycin is recognized for its high sensitization power, reaching prevalences of up to 4%, followed by gentamicin, which can cause a series of toxic reactions on the ocular surface but is rarely reported as a causative agent of ACD. Six different cases of ACD have been reported with positive patch testing to gentamicin.17 Tobramycin is considered a well-tolerated aminoglycoside with low allergenic power; however, in recent years, the number of reports published on sensitization18 to this widely used drug in our setting has been on the rise.
Cross-reactivity between neomycin and other aminoglycoside antibiotics (gentamicin, kanamycin, and tobramycin) has been reported.19,20 However, we should mention that relying on neomycin as a sensitivity marker for this group is insufficient, as there are reports where positivity to tobramycin is not followed by positive patch testing to neomycin.21
ChloramphenicolThis antibiotic is available in eye drops and ointment, used to treat bacterial conjunctivitis and rarely involved in the onset of ACD.22 A total of 7 cases of sensitization to chloramphenicol have been reported in the literature, with 1 of them describing an anaphylactic reaction.23
MacrolidesACD to these antibiotics is extremely rare. Just a few cases of ACD to azithromycin ophthalmic solution have been reported,24 confirmed via patch testing.
VancomycinThis antibiotic is usually spared for serious infections. To date, only 1 case of ACD with a positive patch test has been reported.25 Since there is no marketed presentation, it must be formulated for patch testing.
SulfonamidesDespite being a pharmacological group with some allergenic potential, topical sulfonamides are rarely used nowadays, being replaced by other more effective and safer antibiotics.17
QuinolonesThese are prescribed for infections by gram-positive and gram-negative bacteria. They are notable for their photosensitizing potential.26 No cases of ACD to ophthalmic quinolones have ever been published.
AntiviralsThe most widely used topical ophthalmic antiviral is acyclovir, usually well tolerated for a short application period of 7 to 10 days. ACD to acyclovir is rare, but punctate keratopathy with positive patch testing with 3% acyclovir in vaseline has been reported.17
Antiglaucoma agentsThey are a wide group of ocular preparations, which are used for long periods of time and generally in combination with other agents that also control intraocular pressure, thus increasing the risk of sensitization.27
Prostaglandin analoguesThese are used as first-line therapy in the management of glaucoma. Compared to other antiglaucoma agents, they have minimal systemic effects and very few local adverse events. Prostaglandin analogues-induced ACD is rare. Multiple cases of latanoprost-induced ACD have been reported.28,29 Patients sensitized to latanoprost may tolerate bimatoprost as an alternative.30
Beta-blockersThese are considered second-line drugs in the management of glaucoma. Beta-blockers are currently prescribed as dual therapy along with prostaglandin analogues. This group can cause local and systemic adverse events, including type IV hypersensitivity reactions.31 A retrospective study of ACD to beta-blockers has been reported,32 exposing sensitization to timolol, levobunolol, metipranolol, betaxolol, befunolol, carteolol, and metoprolol.
The cross-reactivity reported among them is unpredictable, although cases of cross-reactivity between timolol and levobunolol have been reported too.33
Carbonic anhydrase inhibitorsTopical and systemic inhibitors of carbonic anhydrase are sulfonamide agents used to reduce intraocular pressure. The most widely used topically is dorzolamide hydrochloride.34 Cases of ACD in the form of conjunctivitis or periorbital dermatitis have been reported with the use of this pharmacological group.35-37 One singe case of ACD to brinzolamide has also been reported.38
SympathomimeticsPhenylephrine39 and dipivefrin40—a prodrug of epinephrine—are considerd α-1 agonists.39 Both are also considered responsible for ACD. Erdmann et al.41 found no cross-reactivity between phenylephrine and other structurally related sympathomimetics, such as epinephrine or ephedrine. However, cross-reactivity has been reported with pseudoephedrine,42 which is widely used in cold drugs. One severe case of fulminant keratoconjunctivitis has been reported with the use of phenylephrine43.
Currently, there are 2 α-2 adrenergic agonists, apraclonidine hydrochloride and brimonidine tartrate. Only 1 case of sensitivity to apraclonidine has been reported so far44 in the form of a very intense reaction in the patch test with the eye drops and pure apraclonidine. There is a 22.7% risk of an allergic reaction to brimonidine in patients with a known allergy to apraclonidine;45 however, this data has not been considered high risk, and the author advocated for brimonidine tartrate as a safe alternative in patients diagnosed with contact allergy to apraclonidine.
ParasympathomimeticsPilocarpine hydrochloride is a miotic agent used to reduce intraocular pressure in closed-angle glaucoma. Several cases of sensitization to pilocarpine46 have been reported, one of them being the photoallergic type.47
Combinations of different groups of antiglaucoma drugsCombinations are common to treat diseases such as glaucoma. The active ingredients and excipients are available in Table 2.
Nonsteroidal anti-inflammatory drugsDiclofenac, ketorolac, ketoprofen, nepafenac, and bromfenac, among others, fall within this pharmacological group. They are widely prescribed to treat intra- and postoperative inflammation in ophthalmic surgery. Cases of sensitization to diclofenac48 have been reported, which can also show cross-reactivity to indomethacin.49 Cases of ACD to ketorolac eye drops have been reported as well.50 Although ketoprofen is known for its sensitizing capacity and is responsible for contact photoallergic reactions,51 no cases of ACD to ketoprofen eye drops have ever been reported.
AnestheticsThe are often used in short-duration surgical procedures, for the extraction of foreign bodies, or as an injectable solution in intraocular procedures. The ester group derivatives—tetracaine,52 proparacaine53, and oxibuprocaine54—have the highest sensitizing power being cross-sensitization a common finding among them.16
Mydriatics and cycloplegicsThese drugs are used to trigger the dilation of the pupil in fundus examination, for anterior chamber surgical procedures, and in some cases, as anti-inflammatory treatment (uveitis, iritis, iridocyclitis). The main sensitizers in this group are phenylephrine41 (30% of all cases), and atropine sulfate.55,56 A few cases of ACD to tropicamide57 and cyclopentolate58 have also been reported. These drugs are mainly associated with periocular dermatitis, eyelid edema, and blepharoconjunctivitis, although cases of systemic ACD have also been reported.59
Antihistamines and cromonesThese drugs are used to treat allergic conjunctivitis. A case of sensitization to sodium cromoglycate eye drops has been reported.60 As for antihistamines, fewer cases of ACD have been reported with the introduction of newer generation antihistamines. Cases of ACD to ketotifen61 and pheniramine maleate have been reported.62 The latter may cross-react with dexchlorpheniramine maleate and chlorpheniramine maleate due to the similarity of its chemical structures.2 One suspected case of ACD to olopatadine63 could not be confirmed through patch testing.
CorticosteroidsCorticosteroids are used to treat non-infectious inflammatory conditions of the anterior segment of the eye, cornea, and conjunctiva. A few examples of these conditions are anterior uveitis, iridocyclitis, keratitis of various etiologies (viral, springtime, allergic), or corneal injuries due to foreign bodies or burns. ACD to corticosteroids is not uncommon, and some studies indicate that the risk of sensitization may be related to the corticosteroid group in question: group 1 (e.g., prednisolone, tixocortol pivalate, budesonide, loteprednol, fluorometholone, and difluprednate), group 2 steroids are most likely to cause contact allergy, while group 3 steroids (e.g., rimexolone, dexamethasone) are associated with a lower risk of sensitization.6 Hydrocortisone, tixocortol pivalate, or budesonide have been described as the most frequently sensitizing agents in the scientific medical literature, although cases have been reported with other corticosteroids such as hydrocortisone derivatives (hydrocortisone acetate, hydrocortisone 17-butyrate), dexamethasone, prednisone acetate, prednisone pivalate, and betamethasone valerate.64 Cross-sensitivity is considered a frequent phenomenon, with cases described of cross-reaction between hydrocortisone and tixocortol pivalate. Sensitivity to corticosteroids should be suspected in patients who do not improve or worsen their ocular dermatitis with corticosteroid treatment.64
AntisepticsCases of periocular ACD with the application of diluted ocular povidone iodine,65 and 0.5% chlorhexidine65 have been reported. Chlorhexidine digluconate is available on the market, while iodinated povidone is not, and its dilutions are not standardized.66 These means that these reactions should be interpreted with caution.
MiscellaneousCases related to natural substances used in ocular products have been described in the literature, such as chamomile,16 a substance from the group of sesquiterpene lactones. Retinoic acid is used in ointments for patients with corneal sequelae from NET formation or pemphigoid, with 1 published case of bilateral periocular dermatitis.67
Vitamin K1 is a lipophilic vitamin used in cosmetics to treat bruises after laser therapy or in periocular makeup. Its non-oxidized form was withdrawn from the market due to its high sensitization potential; however, the oxidized molecule (phytonadione epoxide) is used due to its lower sensitizing power. Cases of periocular and facial dermatitis due to phytonadione epoxide have been reported, possibly due to cross-sensitization due to previous application of a non-oxidized periocular cream.68 Regarding contact lenses, cases of contact allergy to the components of the lenses themselves—hydroxyethyl methacrylate, triethylene glycol diacrylate—have been reported in patients intolerant to such components uch as hydroxyethyl methacrylate, triethylene glycol diacrylate, in patients with intolerance to them.69 Periocular ACD has been described after the application of mitomycin C,70 an antibiotic isolated from Streptomyces caespitosus used as a chemotherapeutic agent to treat ocular surface neoplasms such as squamous cell carcinoma. Cases of ACD due to artificial tears containing N-acetylcysteine,16 a mucolytic used for xerophthalmia, have also been reported. Topical calcineurin inhibitors are used for the long-term management of periocular/eyelid dermatitis, and although sensitization to tacrolimus71 and pimecrolimus72 has been reported, no cases were associated with the ophthalmic formulation.
The marketing of monotherapy ophthalmic products has significantly reduced sensitization to preservatives. In fact, currently, active ingredients lead publications on this topic. Although it is not the ideal scenario, having drugs in single doses frees the product from preservatives and allows the dermatologist to perform patch tests with the suspected contact source knowing that it is the only ingredient, thus providing a more convincing interpretation of the test.
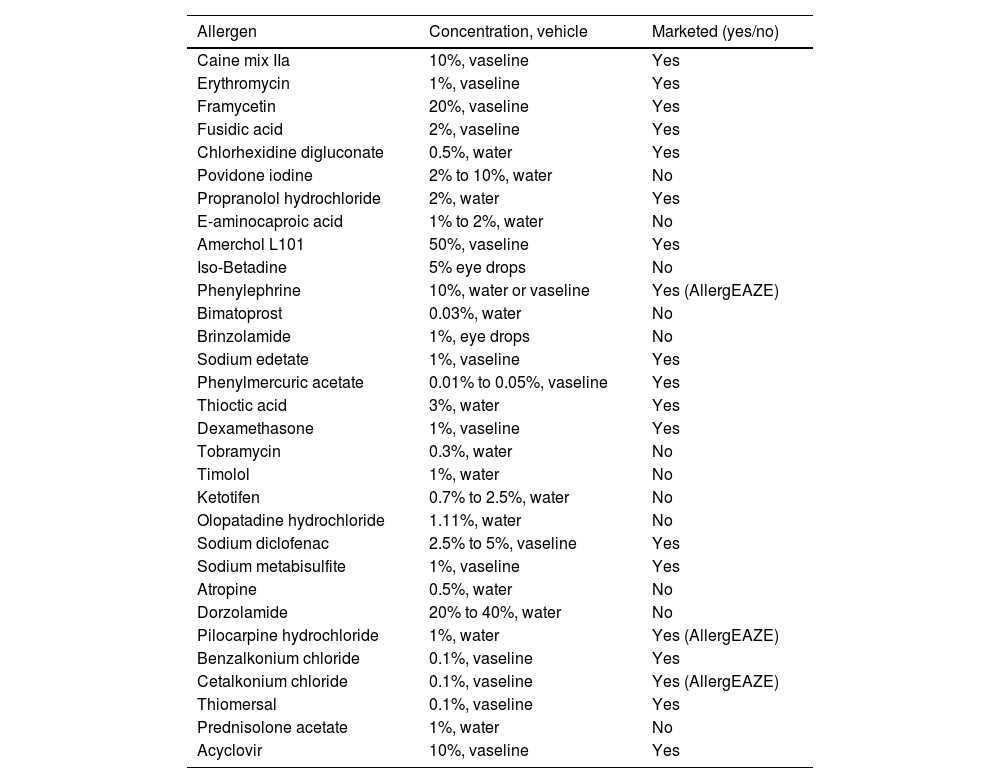
Other sources of allergens responsible for eyelid/periocular dermatitisWe can add the following list of allergens to ophthalmic products: makeup, facial moisturizers, facial cleansers (preservatives, surfactants), shampoo (perfumes, preservatives, surfactants), hair conditioner, false eyelashes (acrylates, colophony), hair dye (paraphenylenediamine), sunscreens, eyelash curlers (nickel, rubber), makeup sponges (rubber), jewelry (metals), acrylic nails (self-applied or ectopic dermatitis), non-acrylic nails (toluene formaldehyde resin), plants (airborne), and connuvial dermatitis (usually due to cosmetic ingredients). Anamnesis remains the key point for an adequate approach to patch testing. Table 3 shows the list of allergens most frequently patched in ACD due to ophthalmic products.
List of allergens published in relation to allergic contact dermatitis of ophthalmological origin.
| Allergen | Concentration, vehicle | Marketed (yes/no) |
|---|---|---|
| Caine mix IIa | 10%, vaseline | Yes |
| Erythromycin | 1%, vaseline | Yes |
| Framycetin | 20%, vaseline | Yes |
| Fusidic acid | 2%, vaseline | Yes |
| Chlorhexidine digluconate | 0.5%, water | Yes |
| Povidone iodine | 2% to 10%, water | No |
| Propranolol hydrochloride | 2%, water | Yes |
| E-aminocaproic acid | 1% to 2%, water | No |
| Amerchol L101 | 50%, vaseline | Yes |
| Iso-Betadine | 5% eye drops | No |
| Phenylephrine | 10%, water or vaseline | Yes (AllergEAZE) |
| Bimatoprost | 0.03%, water | No |
| Brinzolamide | 1%, eye drops | No |
| Sodium edetate | 1%, vaseline | Yes |
| Phenylmercuric acetate | 0.01% to 0.05%, vaseline | Yes |
| Thioctic acid | 3%, water | Yes |
| Dexamethasone | 1%, vaseline | Yes |
| Tobramycin | 0.3%, water | No |
| Timolol | 1%, water | No |
| Ketotifen | 0.7% to 2.5%, water | No |
| Olopatadine hydrochloride | 1.11%, water | No |
| Sodium diclofenac | 2.5% to 5%, vaseline | Yes |
| Sodium metabisulfite | 1%, vaseline | Yes |
| Atropine | 0.5%, water | No |
| Dorzolamide | 20% to 40%, water | No |
| Pilocarpine hydrochloride | 1%, water | Yes (AllergEAZE) |
| Benzalkonium chloride | 0.1%, vaseline | Yes |
| Cetalkonium chloride | 0.1%, vaseline | Yes (AllergEAZE) |
| Thiomersal | 0.1%, vaseline | Yes |
| Prednisolone acetate | 1%, water | No |
| Acyclovir | 10%, vaseline | Yes |
ACD due to ophthalmic products can be a significant diagnostic challenge that requires familiarity with commercially available allergens. Proper preparation of allergens and knowing the most important excipients will be key for proper study through patch testing. Collaboration with the ophthalmologist is important to assist in decision-making regarding treatment changes, avoiding possible drug-related cross-reactions, and eventually leading the dermatologist to provide individualized information of special interest for each particular case.
Conflicts of interestNone declared.