Topical 2,6-diamino-4-piperidinopyrimidine 1-oxide (minoxidil) is probably the most widely used treatment for different types of alopecia. Although the topical solution is usually well tolerated, continued application can sometimes result in itching and irritant contact dermatitis.
A 38-year-old man with no personal or family history of interest was referred to our cutaneous immunoallergy unit for evaluation of an eczematous neck rash that had developed over the previous 2 months.
He had been using topical minoxidil (Regaxidil) to treat alopecia areata for the previous 3 months. He mentioned an episode of alopecia areata characterized by multiple patches in childhood (at the age of 6 years) that had been treated with topical minoxidil at an unknown concentration. The treatment led to complete resolution after 6 months, and until now he had not experienced any other episodes.
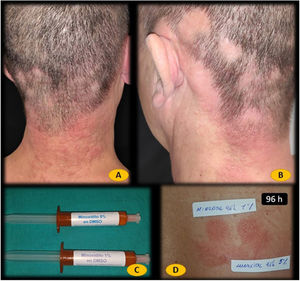
Physical examination revealed erythematous, edematous papules and patches in the occipital region of the neck (Fig. 1A and B). The patient was patch tested with the European Baseline Series (Chemotechnique Diagnostics), 30% aqueous propylene glycol, and minoxidil 1% and 5% in dimethylsulfoxide (DMSO) (Fig. 1C). The results were interpreted using the criteria of the International Contact Dermatitis Research Group. Readings were performed on days 2 and 4. The reaction to minoxidil 1% and 5% in DMSO was negative on day 2 (Fig. 1D) and positive (++/+++) on day 4 (Fig. 1E). Patch tests with minoxidil 1% and 5% in DMSO were negative in 12 controls.
The patient was diagnosed with allergic contact dermatitis due to minoxidil. The product was discontinued and the lesions disappeared completely within a week. No signs of hair regrowth were observed, despite the inflammatory reaction to contact dermatitis from minoxidil.
Few cases of allergic contact dermatitis due to minoxidil have been reported.1 Other contact reactions related to the topical application of this product include pustular allergic contact dermatitis,2 occupational allergic contact dermatitis, and pigmented contact dermatitis.3 A case of persistent allergic patch test reactions to minoxidil manifesting as cutaneous lymphoid hyperplasia has also been reported.4
Choice of vehicle is important for patch testing. Direct testing may generate doubts, as topical minoxidil solution contains propylene glycol. That is why we chose DMSO as a vehicle, as it favors penetration of the active ingredients and is valid for both hydrophilic and lipophilic substances.
The recent introduction of oral minoxidil has revolutionized the treatment of certain forms of alopecia (androgenetic alopecia).5 Identifying patients sensitized to topical minoxidil could be important, as certain patients with alopecia may require oral minoxidil to control hair loss. Although oral minoxidil can probably be safely used, the possibility of systemic contact dermatitis cannot be fully ruled out, despite early reports suggesting it is unlikely.6,7
Conflicts of InterestThe authors declare that they have no conflicts of interest.