A 72-year-old man was evaluated in the emergency department of a different center for the development of generalized, confluent erythematous macules and papules on the extremities, with a morbilliform appearance, marked pruritus, and occasional blisters. The mucosae were spared, and no other systemic signs were present. The lesions had progressed gradually over approximately 10 days. The patient was on chemotherapy (carboplatin and paclitaxel) and pembrolizumab for stage IV squamous non-small cell lung cancer with PD-L1 expression<1%. Pembrolizumab (200mg) had been introduced 10 days earlier during the 3rd chemotherapy cycle, with no other changes in medication.
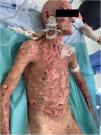
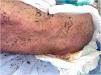
The patient was diagnosed with Stevens–Johnson syndrome (SJS), underwent skin biopsy, and was started on prednisone 1mg/kg/d with an initially favorable response and clinical improvement the next week. However, 19 into chemotherapy, he started experiencing clinical deterioration, with involvement of 60% of the body surface area, a SCORTEN score of 4 (estimated mortality 62.25%), bullae appeared, along with painful epidermal detachment, hemorrhagic crusts, hematuria, and significant oral mucositis (Figs. 1 and 2).
Given the poor clinical progression, a 2nd skin biopsy was performed due to suspicion of toxic epidermal necrolysis (TEN). Since the patient had not experienced any adverse effects with the first 2 chemotherapy cycles, pembrolizumab was suspected as the causative agent.
Methylprednisolone was increased to 1.5mg/kg/d for 3 days in pulses, and a single dose of etanercept 50mg was administered. Wound care every 48h included aqueous chlorhexidine, paraffin gauze dressings, clobetasol, and topical gentamicin to prevent secondary infection. Histopathology was consistent with the presumed diagnosis, revealing complete epithelial denudation, vacuolar interface damage in the residual follicular adnexa, and absence of immune deposits on direct immunofluorescence in both biopsies.
On day 26 following pembrolizumab administration, the patient again worsened, with 80% total body surface area detachment; cyclosporine 3mg/kg/d was added. Over the next 3 weeks, the cutaneous lesions re-epithelialized progressively until achieving near-complete resolution (Fig. 3), allowing gradual tapering of immunosuppression. One month after discharge from our service, the patient died due to complications related to his malignancy.
Immune checkpoint inhibitors (ICIs) are a novel class of anticancer agents that modulate T-cell activation. Under physiologic conditions, programmed cell death protein 1 (PD-1), its ligand PD-L1, and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) attenuate T-cell activation, supporting tolerance and homeostasis—mechanisms exploited by tumor cells to evade immune surveillance.1 In contrast, inhibition of these pathways triggers chronic inflammation that may result in immune-related adverse events (irAEs),1 which can affect any organ system and involve the skin in up to 40% of patients.2
To classify reaction severity, the U.S. National Cancer Institute developed the Common Terminology Criteria for Adverse Events (CTCAE),3 in which differentiation between grade 3 and grade 4 is essential because grade 4 requires permanent discontinuation of the offending agent—this includes TEN.4 The mechanism by which anti-PD-1 agents may induce SJS/TEN appears to involve cytotoxicity with keratinocyte apoptosis and CD8+ lymphocytic infiltration at the dermoepidermal junction.5
Morbilliform eruptions are a common finding during anti-PD-1 therapy, occurring in approximately 16.7% of patients treated with pembrolizumab and 14.3% with nivolumab,5 but <1% progress to TEN.4 Clinical presentation resembles classic TEN, with a prodrome of fever, constitutional symptoms, and generalized eruption progressing to blistering, mucosal involvement, and a positive Nikolsky sign,4 though often with a slower course. The longer latency between drug administration and symptom onset is believed to relate to pembrolizumab prolonged half-life and delayed attainment of steady-state levels vs conventional drugs.1,6 Reported cases in the literature describe a median latency of 11 weeks, ranging from 2 to 28 weeks after drug initiation.1,6
In patients on ICIs who develop a slowly progressive exanthem unresponsive to topical or systemic corticosteroids, the possibility of progression to SJS/TEN should be considered because early treatment—such as plasmapheresis—may be beneficial.1 Although the use of corticosteroids and immunosuppressants in classic TEN remains controversial,7 IV corticosteroids are strongly recommended in cases associated with ICIs, having been used in all published cases, often in combination with cyclosporine or intravenous immunoglobulin.6,8,9 Moreover, anti-TNF-α inhibitors such as etanercept may reduce the required corticosteroid dose and thereby minimize adverse effects.10
Conflict of interestThe authors declare that they have no conflict of interest.