The combination of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI) is widely used as a preservative in cosmetics, household, and industrial products. Furthermore, MI at a concentration of 100ppm has been permitted in cosmetic products since 2005. Recently, a considerable increase in cases of contact dermatitis to both MCI and MI have been noted, and this warrants closer monitoring by relevant authorities and, probably, stricter legislation. In fact, MI at a test concentration of 2000ppm was recently included in the European baseline patch test series. The clinical manifestations of allergy to MCI/MI and MI are highly variable and diagnosis is often missed. In the standard patch test series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC), MCI/MI is tested at 100ppm, but at this concentration, up to 50% of cases might go undetected. Furthermore, our data indicate that MCI/MI at 200ppm would make it possible to diagnose more cases of contact allergy to MI. To improve the diagnosis of contact allergy to MCI/MI and MI, we believe that the test concentration of MCI/MI should be increased to 200ppm in the GEIDAC standard series and that MI should be added in the GEIDAC standard series.
La combinación de metilcloroisotiazolinona (MCI) con metilisotiazolinona (MI) es ampliamente empleada como conservante tanto en productos de higiene y domésticos como industriales. Desde 2005 está permitido el uso de MI a 100ppm en cosméticos. En los últimos años se está detectando un aumento considerable de los casos de dermatitis de contacto a los 2 conservantes, por lo que es necesaria una monitorización estrecha por parte de las autoridades y probablemente unas medidas legislativas más estrictas. De hecho, MI a 2000ppm se ha incluido recientemente en la batería estándar Europea. La forma de presentación clínica es muy variable, y en ocasiones es difícil sospechar una alergia a MCI/MI y MI. MCI/MI se testa en la batería estándar del GEIDAC a 100ppm, pero con esta concentración se podría estar dejando de diagnosticar hasta la mitad de los casos. Además, según nuestros datos MCI/MI a 200ppm permite diagnosticar más casos con alergia a MI. Para llegar a un buen diagnóstico consideramos que se debería aumentar la concentración del parche de MCI/MI a 200ppm e incluir MI en la batería estándar del GEIDAC.
The combination of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI), which is more commonly known as Kathon CG, has been and continues to be widely used as a preservative in cosmetic, household, and industrial products. After formaldehyde releasers, MCI/MI is the second most common cause of contact allergy to preservatives in Europe.1 During the 1980s, a large number of patients were reported to be allergic to MCI/MI. Since it was originally thought that the allergenicity of the combination lay in MCI, use of MI alone was authorized at high concentrations in cosmetic products in 2005. The first cases of MI-induced allergic contact dermatitis soon began to emerge.2 We are currently witnessing a considerable increase in the number of patients who are allergic to MCI/MI and MI,3 and some authors have warned of a possible epidemic.3,4 MI was named allergen of the year in 2013 by the American Contact Dermatitis Society and included in the European standard series.5 Moreover, it is very likely that a considerable number of cases of allergy to MCI/MI or MI go undetected with the concentration of MCI/MI used in the patches of the European and Spanish standard series.6,7
The primary objective of this article is to provide an update on allergic contact dermatitis caused by MCI/MI and MI. We review the main sources of exposure and current legislation on the use of these allergens in both domestic and industrial products. We also provide available clinical and epidemiological data on allergy to MCI/MI and present our approach to diagnosis and treatment.
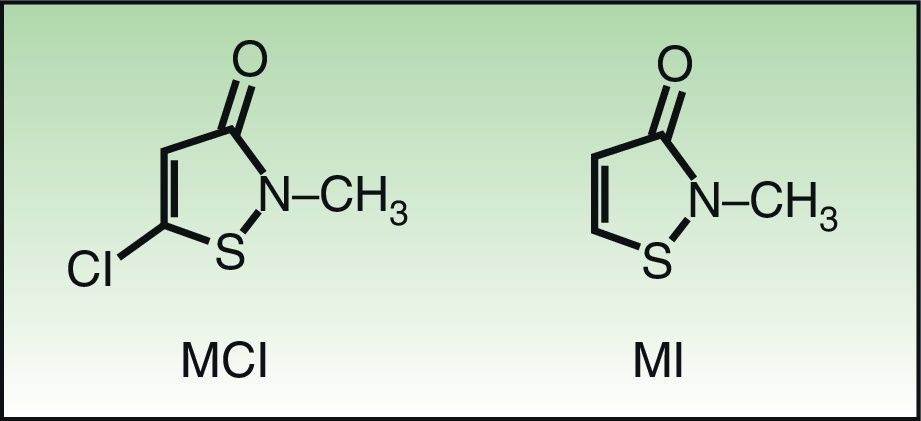
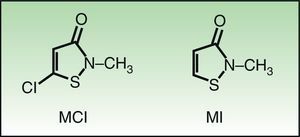
Molecular Structure and Sensitization Studies5-Chloro-2 methyl-4-isothiazolinone/2-methyl-3,4-isothiazolinone (MCI/MI) and 2-methyl-3,4-isothiazolinone (MI) are members of the isothiazolinone group. Isothiazolinones are heterocyclic organic components used as biocides owing to their marked antibacterial and antifungal activity within a wide range of pH values. MCI/MI is used as a preservative at a ratio of 3:1. MI is less potent than MCI/MI and, as such, must be used at higher concentrations in order to be sufficiently biocidal. The only difference in the molecular structure of these substances is the presence of a chloride in MCI that leads to a different type of interaction with the allergenic proteins. The resulting formation of a very electrophilic and, therefore, very reactive intermediate, makes MCI more allergenic.8 (Fig. 1) The results of studies in animals and healthy volunteers show that MCI is 30 times more potent than the nonhalogenated form MI and that MI was a mild-to-moderate sensitizer with no ability to induce respiratory sensitization. In a further 2 studies in which concentrations of MI of 100-600ppm were analyzed, healthy volunteers could only be sensitized at concentrations of 600ppm or higher.9 In the murine local lymph node test, MI was classified erroneously by some authors as a moderate sensitizer. It was subsequently shown that the results pointed to MI as a strong sensitizer.10 Scientific committees and evaluators from the United States of America and the European Union concluded that MI at 100ppm was safe for use in cosmetic products. However, despite the so-called safe concentrations of MI, postmarketing studies revealed cases of allergy to this preservative in cosmetic products.1,2,9,11 Finally, the relatively high incidence detected in studies on patients with eczema performed in skin allergy units highlights the marked allergenicity of these substances.12,13
SourcesMCI/MI and MI are widely used in consumer and in industrial products.
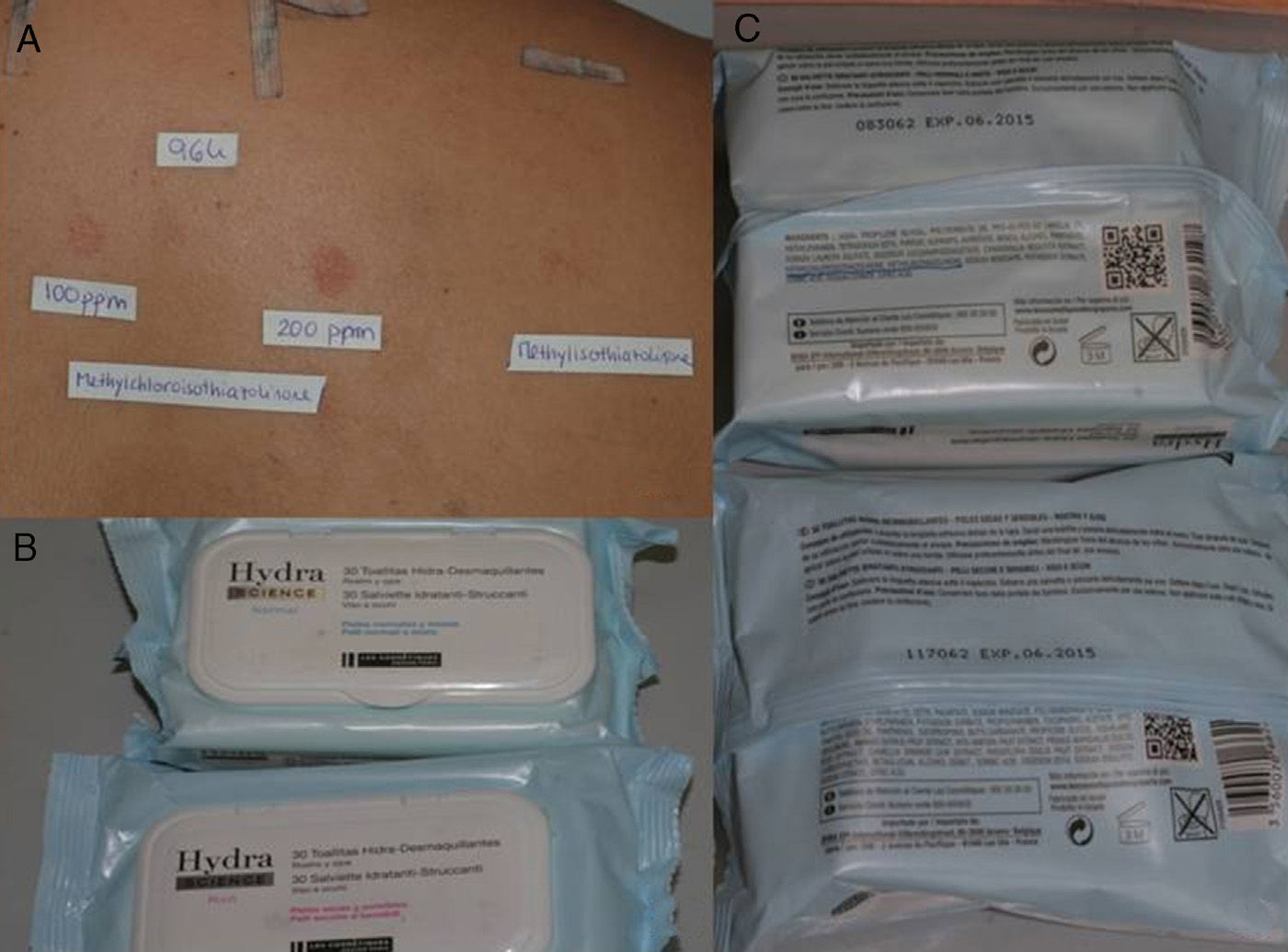
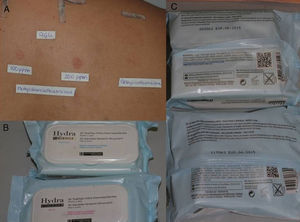
Consumer ProductsThe preservatives are found in cosmetics and personal hygiene products (rinse-off and leave-on) such as soaps, gels, shampoos, leave-on products for scalp care, sunscreens, deodorants, moisturizing creams, intimate wipes, baby wipes, and makeup remover wipes (Fig. 2). They are also present in cleaning products such as washing up liquid, detergents, stain removers, window cleaning solution, grease remover, and air fresheners.
A, Patient with eyelid eczema and positive results to methylchloroisothiazolinone/methylisothiazolinone. B and C, The patient linked her eczema to makeup remover wipes and, therefore, switched to wipes of the same brand that did not contain methylchloroisothiazolinone/methylisothiazolinone. The eczema resolved.
In a study performed in 2010 in Denmark, a review of 1272 cosmetic product labels revealed that MCI/MI was mentioned on 132 (10.4%).9 In contrast, MI was present as a preservative in only 19 products (1.5%), of which 70% were rinse-off; wet wipes accounted for half of the leave-on products. Analysis of the concentrations of MI revealed that 74% contained more than 50ppm and 16% contained more than 95ppm. MI was not detected in 2 cases. In a similar study in Denmark in 2013, a review of 1795 labels from cosmetic products revealed that 60 contained MI (3.3%), which is more than double the figure recorded in the previous study. Furthermore, since only 37% were rinse-off products, this preservative seems to be more widely used in leave-on products.4 In a study on sources by Magnano et al.,14 who focused on household cleaning products, MCI/MI was present in 35.7% and MI in 10.3%. In Spain, sources have not been studied, although our impression is that the presence of these preservatives in cosmetic and cleaning products is greater (Fig. 3).
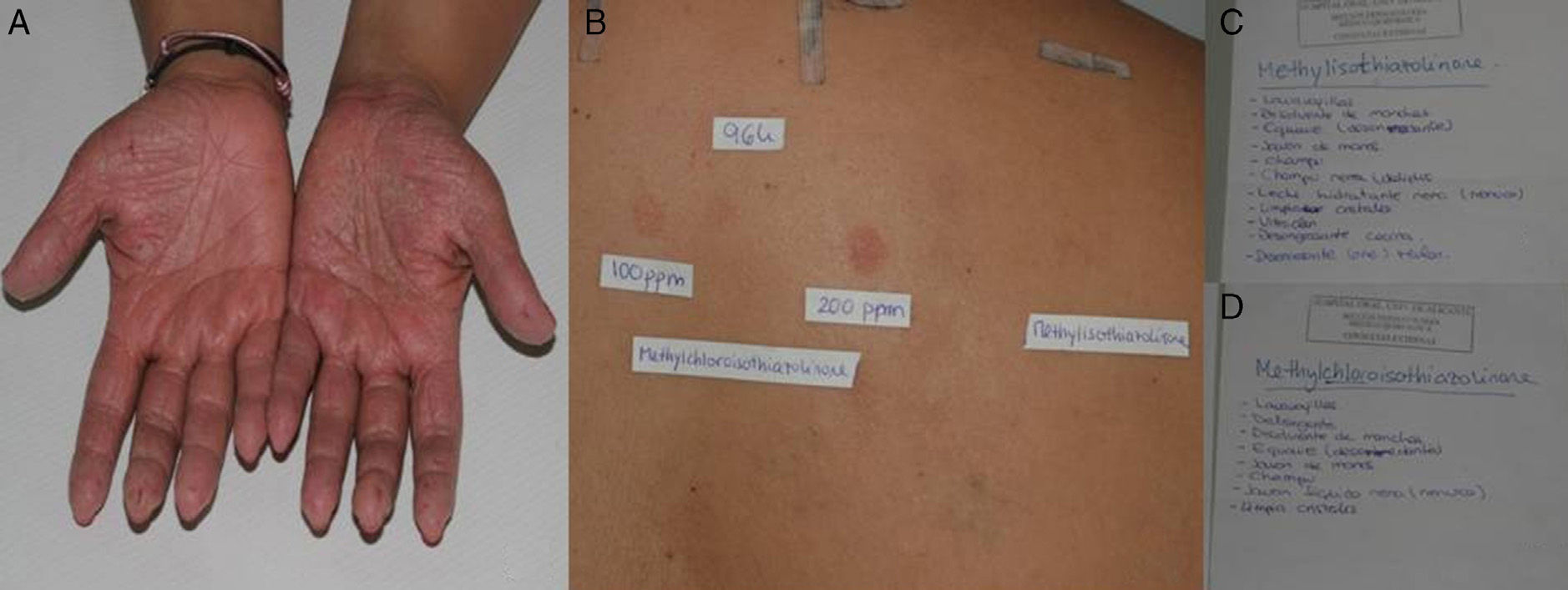
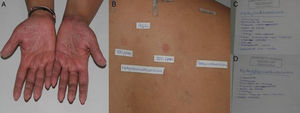
A and B, Patient with allergic contact dermatitis on the hands caused by methylchloroisothiazolinone/methylisothiazolinone. C and D, The patient provided the names of a large number of cosmetic and domestic products containing methylchloroisothiazolinone and methylisothiazolinone.
MCI/MI can be found in the industrial setting, mainly in workplace handwashing soap, water-based paints, lacquers, toners, printer ink, adhesives, coolants, and oils. In recent decades, there has been a shift from solvent-based paints to water-based paints, which are more environmentally friendly. Consequently, it became necessary to preserve paint with MCI/MI, and later MI, the most commonly used preservatives.15 The risk of sensitization to paint not only affects painters and paint factory workers, but also domestic users.
In 2002 and 2005, Flyvhom16 carried out a review of preservatives used in chemical products registered in the Danish product registration database (PROBAS) and found that MCI/MI was present in 1872 products in 2002 and in 3563 products in 2005. During both periods, paints and lacquers (26.6% in 2002 and 60.1% in 2005) were the main sources. There are no studies on the source of MI in industrial products, and currently available information is from different paint manufacturers who claim that the concentrations of MI used range from 100ppm to 270ppm.16
Epidemiology and Legislation (Table 1)MCI/MI has been used as a preservative in Europe since the 1970s, and the first cases of occupational contact dermatitis were reported in the 1980s. Reports of cases in domestic users soon followed, with sensitization rates of 3-8% being reached in a few years (Table 1).17 In 1989, this “epidemic” had led the EU to limit concentrations of MCI/MI to 15-55ppm in industrial products and 15ppm in cosmetic products. For some authors,18 this measure has been effective; for others,11,17 however, it has not succeeded in sufficiently reducing rates of sensitization to MCI/MI, which have remained at 1.8-4.4% for the last 20 years.6,11,12,18,19 Furthermore, during the last 4 years there has been an alarming increase in sensitization to MCI/MI (4-5%).3,18 We confirmed this finding in our department, where the rate increased from 4.49% during the period October 2006-September 20086 to 10% in October 2011-September 2013 (Table 2). This increase could be due to the use of MCI/MI instead of methyldibromo glutaronitrile, which was banned in cosmetics in 2008.3 Another possible explanation is the introduction of MI at high concentrations with primary sensitization to MI and subsequent cross-reactivity with MCI/MI.12
Legislation.
| Directive 89/174/EEC of the European Commission, dated February 21, 1989 limits the use of methylchloroisothiazolinone/methylisothiazolinone in industrial products to 15-55ppm. Concentrations above 15ppm must be displayed on the label. Similarly, a limit of 15ppm is established for cosmetic and household products (rinse-off and leave-on) and must be specified on the label (embodied in Spanish Royal Decree 1599/1997).In the United States of America, the limit for cosmetic products is set at 15ppm in rinse-off products and 7.5ppm in leave-on products. In countries such as Japan, the use of methylchloroisothiazolinone/methylisothiazolinone is prohibited in leave-on products.Directive 2005/42/EC of the European Commission dated June 29, 2005 authorizes the use of methylisothiazolinone in cosmetic and household products up to 100ppm with mandatory labeling (embodied in Spanish Order SCO/747/2006, dated March 9).No limits have been set for the methylisothiazolinone concentration in industrial products. Specification on the label is not mandatory. |
Prevalence Of Allergy To Methylchloroisothiazolinone (MCI)/Methylisothiazolinone (MI) And Progress Over Time.
MI alone was launched as a preservative for use in industrial products in 2000; the first cases of occupational allergic contact dermatitis to MI were reported in 2004. Since MI was thought to be a weak sensitizer, the EU authorized its use in cosmetic products at 100ppm in 2005. In 2010, 7 cases of allergic contact dermatitis to MI in domestic users were reported,2 and subsequent reports followed. The prevalence of sensitization to MI ranges from 0.5% to 6% depending on the study,1,4,5,18 and the trend is rising (Table 3).3,12 Some authors warn of a possible epidemic of allergic contact dermatitis to MI in cosmetics; this is particularly alarming given the short time the product has been on the market,3–5 and urgent preventive measures and more in-depth studies have been requested.3,18,20 Consequently, MI was recently included in the European standard series.5
In summary, the limitations imposed are not sufficient to prevent dermatitis in patients who are already allergic to MCI/MI and MI,21 and data seem to indicate that the measures are insufficient to prevent new sensitizations. Therefore, several working groups believe that legislation should be stricter.3,5,20,22,23
Clinical ManifestationsGiven that patients are exposed to MCI/MI and MI on a daily basis, allergic contact dermatitis generally manifests as subacute or chronic eczema. It can appear on any part of the body, although the hands are the most frequently affected site, both at work and at home and in adults and children alike (Fig. 3).1,4,24 The eczema very often mimics homemaker-type chronic irritant dermatitis. Eczema is also frequently found on the face, especially in younger women, because of exposure to cosmetic products (Fig. 2).3 MI-allergic patients tend to be older than MCI/MI-allergic patients.4 There have been reports of several cases of perianal eczema caused by exposure to MCI/MI and MI in wet wipes, where sensitization could be enabled by occlusion in nonkeratinizing epithelium.13,23
Airborne dermatitis has been reported in painters and paint industry workers, although it has also been reported in domestic users whose house was recently painted. In some cases, the patients presented respiratory symptoms requiring admission to hospital.2,20 Symptoms can last several weeks, indicating that MCI/MI and MI are released into room air during this time.
We observed that many patients presented patchy diffuse eczema (mainly on skin folds) that mimic atopic dermatitis and are referred to our clinic because of poor response to treatment.
Finally, sensitization has been reported after massive burns with high concentrations of these components. Monsálvez et al.21 published the case of a worker who experienced a burn with MCI/MI and developed widespread eczematous lesions in under 120hours. The results of patch tests were positive for MCI/MI.21
DiagnosisCorrect diagnosis requires compatible clinical manifestations and history, as well as positive patch test results with present relevance. The history should include the cosmetic products used, type of work, aggravating factors (eg, occupation), and changes affecting lifestyle (eg, birth of a child or recent housepainting). We should be on the alert for possible allergy to MCI/MI in at-risk occupations such as housepainter, recently painted houses, and in cases where the patient suspects a potentially allergenic cosmetic or household product (rinse-off and leave-on). The hands are very often involved, especially in occupational disease. If eczema affects the perianal region, we should suspect wet wipes. Airborne eczema in patients whose house has recently been painted is highly suspicious. Patch testing will enable us to confirm the diagnosis of allergy to MCI/MI. The ideal allergen concentration is that which enables us to detect the greatest number of cases of allergic contact dermatitis to a compound without causing irritant reactions or active sensitization.
MCI/MI is included in the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) and in the European series at 100ppm, although in some, such as the English or Swedish series, it is tested at 200ppm.7 In the TRUE test panel, the concentration is equivalent to 150ppm.
The optimal concentration is open to debate. A growing number of authors feel that MCI/MI should be tested at 200ppm, since this would reveal 46-50% more cases of allergic contact dermatitis to MCI/MI without causing irritant reactions.6,7 During the period 2006-2008 in our department, the Chemotechnique series, which includes MCI/MI at 200ppm, enabled us to detect 24% of MCI/MI-allergic patients to MCI/MI who would have gone undiagnosed with the standard series.6 Even more cases can be detected at 300ppm, although the risk of primary sensitization is high.
MI at 2000ppm in water was recently included in the European standard series with the aim of detecting all allergies to MI, carrying out follow-up in different areas, and ensuring the availability of information to plan future legislative measures. The European Society of Contact Dermatitis and the European Environmental and Contact Dermatitis Research Group recommend using MI in water at 2000ppm (15-μL volume) for a Finn Chamber measuring 8mm in diameter (final dose of 60μg/cm2). The choice of 2000ppm was based on previous studies in which no active sensitizations were detected with this concentration and on experience gained by studying approximately 8500 patients.5,22
Since October 2011, we have been testing concentrations of MCI/MI at 100ppm and 200ppm and MI at 2000ppm. Our findings are as follows:
- a)
Positive result to MCI/MI at 100 and 200ppm and negative result with MI: allergy to MCI detected with the allergen from the standard series.
- b)
Positive result to MCI/MI at 100 and 200ppm and positive result with MI: allergy to MI detected with the allergen from the standard series.
- c)
Positive results to MCI/MI at 200ppm and negative result for MCI/MI 100ppm and MI: false negative for the standard series that would be detected by increasing the concentration of MCI/MI in the standard series to 200ppm.
- d)
Positive results to MCI/MI at 200ppm and MI and negative result for MCI/MI at 100ppm: allergy to MI that would have gone undetected with the standard series.
- e)
We detected no cases that were positive for MI and negative for MCI/MI at 200ppm. All cases of allergy to MI were detected with MCI/MI at 200ppm, which corresponds to an MI concentration of 50ppm. However, we only observed positive results to 100ppm in 68.2% of these cases.
In summary, a 200-ppm MCI/MI patch not only makes it possible to detect more cases of allergy to MCI/MI, but it would also enable cases of allergy to MI to be detected. In our study, a quarter of MCI/MI-allergic patients would have gone undetected if the patch from the standard series had been used. However, we cannot be sure whether the patient is allergic to MCI, MI, or both. If both allergens are involved, it is very difficult to determine which is the primary allergen that triggered the cross-reaction. Given the greater allergenic potential and more widespread use of MCI, many authors consider MCI the most likely primary allergen. They even attribute the increased number of cases of allergy to these preservatives to increased exposure to MI in patients who were previously sensitized to MCI/MI. Furthermore, the frequency of concomitant reactions to MI in MCI/MI-allergic patients is on the rise, increasing from 37% during 2006-2009 to 76% during 2010-2012 in Denmark.4 This finding, together with the fact that the allergenic potential of MI is greater than initially thought,10 points to a more prevalent role for MI as a primary allergen. If MCI and MI are not assessed separately, it could prove extremely complicated to identify the allergen with any degree of accuracy.1,9 Nevertheless, knowing which of the two a patient is allergic to has no practical application, since cross-reactivity between them means that the patient is recommended to avoid both, despite being allergic to only 1.
If the results of patch testing are positive, it is necessary to determine relevance in order to identify the causal agent. In our department, when we detect positivity in the first reading (48hours), we write the names MCI and MI on 2 pages. We ask the patient to look at the label and note the cosmetic or domestic products he/she has contact with that contain these agents so that we can more easily determine the relevance (Fig. 3).
TreatmentWe recommend that patients avoid both rinse-off and leave-on products. Owing to cross-reactivity in a patient diagnosed with allergic contact dermatitis to MI, MCI/MI, or both, it is important to avoid these agents at home and at work. The recommendations we provide to these patients are set out in Table 4.
Recommendations For Patients With Allergy To Methylchloroisothiazolinone And Methylthiazolinone.
| Methylchloroisothiazolinone and methylthiazolinone are both widely used preservatives. They can be found in cosmetic products and household cleaning products, although they are also used in industrial products such as paint and lacquer.Try to avoid these products at home, at work, in restaurants and hotels, and at the homes of friends and relatives.Products containing one or the other allergen should be avoided, even in cases of allergy to only 1 of the 2 components.The same brand may include products that contain these preservatives and other products that do not. Therefore, loyalty to a specific brand is not recommended, and all labels should be checked (Fig. 2). |
Primary preventive measures are aimed at avoiding sensitization. In our opinion, legislation should be modified to ensure stricter limits for authorized concentrations of MCI/MI and MI. Some authors recommend reducing the concentration to 7.5ppm in leave-on products, as is the case in the USA, or even prohibiting them, as is the case in Japan, since they are considered to be more allergenic than rinse-off products.1,11 Repeated proposals have also been made to reduce the authorized concentration of MI in cosmetic products,3 owing to the alarming increase in cases of allergic contact dermatitis to MI. We believe that it is imperative to limit the concentration of MI in industrial products and that labeling should be mandatory.
It would also be interesting to introduce new preservatives or less allergenic concentrations. Phenoxyethanol rarely causes allergic contact dermatitis, although it generally has to be combined with other preservatives owing to its low antimicrobial power.1,22 The combination of phenoxyethanol 0.2-0.4% and MI at 5ppm has proven suitable as a preservative in cosmetic products, and the addition of phenoxyethanol 0.4% to MI in patch tests in patients sensitized to MI does not increase the number of positive results.22 Therefore, combining phenoxyethanol and MI seems to be an interesting option for reducing the number of sensitizations.
ConclusionsThe preservatives MCI/MI and MI are widely used in both domestic and industrial products and have been shown to be relevant allergens. Allergy to these agents has become considerably more frequent in recent years. Clinical presentation is very variable, although allergy should be ruled out in cases of eczema affecting the hands or face and in cases of undiagnosed generalized eczema. In order to confirm the diagnosis, we think that the concentration used in the MCI/MI patch should be increased to 200ppm and that MI should be used in the standard series of the GEIDAC. Similarly, the relevant authorities should ensure close monitoring and probably stricter legislation.
Ethical DisclosuresProtection Of Persons And Animals:The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right To Privacy And Informed ConsentThe authors declare that no private patient data are disclosed in this article.
Conflicts Of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Leiva-Salinas M, Francés L, Silvestre J. Actualización En La Dermatitis De Contacto Alérgica Por Metilcloroisotiazolinonametilisotiazolinona Y Metilisotiazolinona. Actas Dermosifiliogr. 2014;105:840–846.