Chondrodermatitis nodularis helicis (CNH) is a painful idiopathic degenerative condition involving the skin and cartilage of the helix or antihelix of the ear. Topical nitroglycerin 2% is a relatively recent treatment option for CNH that has produced good results, although with adverse effects (17% of cases). The use of a lower concentration would probably achieve similar results with fewer adverse effects. The aim of this study was to evaluate the effectiveness and safety of topical nitroglycerin 0.2% in the treatment of CNH.
Material and methodsWe performed a retrospective observational study of patients treated in 2 Spanish hospitals between 2012 and 2014. The effectiveness of treatment was determined by clinical photography and assessment of symptoms using a verbal numerical rating scale.
ResultsOf the 29 patients treated, 93% showed clinical improvement. In the group of responders, mean treatment duration was 1.8 months and mean follow-up was 5.9 months. Overall tolerance was good in all cases.
ConclusionTopical nitroglycerin 0.2% is an effective and well-tolerated conservative treatment option that improves the appearance of lesions and provides symptomatic relief in the majority of patients with CNH.
La condrodermatitis nodular del hélix (CNH) es un proceso idiopático, degenerativo y doloroso que afecta a la piel y al cartílago del hélix o del antéhelix.
Recientemente se ha descrito la utilidad de la nitroglicerina (NTG) tópica a 2% en el tratamiento de la CNH con buenos resultados, aunque con una tasa de efectos secundarios en el 17% de los casos. Es probable que a una concentración menor se pueda mantener el mismo efecto mejorando la tolerancia.
Nuestra finalidad fue evaluar la efectividad y seguridad de la NTG tópica al 0,2% para el tratamiento de la CNH.
Material y métodosSe llevó a cabo un estudio observacional retrospectivo entre los años 2012 y 2014 en 2 centros hospitalarios españoles. La efectividad se determinó a través de la evaluación clínica, realizada mediante seguimiento fotográfico, y de los síntomas de la lesión, medido mediante una escala numérica verbal.
ResultadosVeintinueve pacientes recibieron el tratamiento, de los cuales el 93% manifestaron una mejoría clínica con una duración media del tratamiento de 1,8 meses y un tiempo de seguimiento medio en los pacientes respondedores de 5,9 meses. La tolerancia fue buena en general en todos los casos.
ConclusiónLa NTG tópica al 0,2% se plantea como una opción conservadora, efectiva y bien tolerada para el tratamiento de la condrodermatitis nodular del hélix que mejora tanto la apariencia clínica como la sintomatología en la mayoría de los pacientes.
Chondrodermatitis nodularis helicis (CNH) was first described by Wrinkler1 in 1915. It is an inflammatory lesion found mainly on the helix or antihelix. The main etiologic and pathogenic factors are exposure to sunlight and repeated local injury.2
Treatment can be medical or surgical and takes several forms, with varying and often unsatisfactory results that frequently lead to relapse. Few controlled studies show the superiority of one treatment over another.
General postural measures are a key aspect of treatment, although this approach is often insufficient or at least does not lead to rapid improvement of symptoms. In recent years, several novel noninvasive approaches have been reported for the management of CNH, including photodynamic therapy or 2% topical nitroglycerin, which proved successful, with a 60% complete response rate and side effects in approximately 17% of cases.3–7 However, it seems likely that topical nitroglycerin administered at a lower concentration could maintain the same clinical response while improving tolerance.
ObjectiveTo determine the safety and effectiveness of 0.2% topical nitroglycerin as an alternative treatment for CNH.
Material and MethodsWe performed a retrospective observational study between 2012 and 2014 at 2 Spanish hospitals: Hospital Unversitario 12 de Octubre in Madrid and Hospital de Manises in Valencia.
Treatment was started using a compounded formulation of 0.2% topical nitroglycerin in white petrolatum.
The standard procedure involved an initial application of a thin layer of the formulation over the lesion and 0.5cm of the surrounding healthy skin once daily for the first 10 days. If the patient tolerated the treatment, then the product was applied every 12hours until the symptoms had resolved.
The patient was also given general instructions on mechanical options to relieve pressure on the affected area such as postural measures and special anatomical pillows.
The effectiveness of treatment was determined by physical examination, photographs, and a pain questionnaire based on a verbal numerical scale before initiating treatment, 1-2 months after initiating treatment, and every 3 months after treatment had finished. Complete response was defined as resolution of the initial symptoms with absence of pain (measured as 0/10 on the verbal numerical scale) and scarring or disappearance of the lesion. Partial response was defined as a reduced pain score (improvement in the numerator of the fraction for the verbal numerical scale with respect to baseline) and improved appearance of the lesion (reduced size and/or erythema) with respect to baseline. Nonresponse was defined as no pain relief (ie, no variation in the pain score), no improvement in the appearance of the initial lesion at the first visit after starting treatment (1-2 months later), or both.
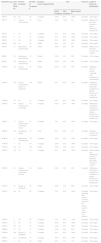
ResultsWe treated 29 patients with 0.2% topical nitroglycerin (20 women and 9 men with a mean age of 67.8 years [range, 29-85 years]). Approximately half of the patients (48%) had previously received 1 or more treatments combined with general measures for reducing pressure on the lesion, with no response (primary failure) or subsequent relapse (secondary failure). The remaining patients received the compounded formulation as their initial treatment. The clinical, demographic, and efficacy data are summarized in Table 1.
Demographic and Clinical Data, Response to Treatment, Tolerance, and Follow-up.
| Patient/Sex/Age | Time Since Onset, mo | Previous Treatment | Duration of Treatment, d | Response, None/Complete/Partial | Pain | Tolerance | Length of Follow-up, mo/Remarks | ||
|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Improvement, % | |||||||
| 1/W/74 | NA | No | 30 | Complete | 10/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 2/W/79 | 12 | Topical corticosteroids | 30 | None | 10/10 | 10/10 | 0% | Excellent | 0/Removal of the lesion because of lack of efficacy of nitroglycerin |
| 3/W/63 | 6 | No | 15 | Complete | 4/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 4/W/69 | 7 | No | 30 | Complete | 5/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 5/W/83 | 2 | No | 30 | Complete | 9/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 6/W/85 | NA | No | 30 | Partial | 6/10 | 1/10 | 83% | Excellent | 6/No relapse |
| 7/W/55 | 24 | Intralesional corticosteroids | 30 | Partial | 10/10 | 8/10 | 20% | Excellent | 6/Removal of the lesion owing to poor response |
| 8/M/56 | 36 | Intralesional corticosteroids | 30 | Partial | 10/10 | 2/10 | 80% | Excellent | 12/No relapse |
| 9/M/29 | 120 | Intralesional corticosteroids | 45 | Partial | 10/10 | 2/10 | 80% | Excellent | 17/No relapse |
| 10/W/53 | 142 | Topical corticosteroids | 45 | Partial | 10/10 | 2/10 | 80% | Excellent | 18/Relapse after 18 months coinciding with reinitiation of chemotherapy |
| 11/M/74 | NA | Topical corticosteroids, surgery (2 operations) | 105 | Partial | 8/10 | 0/10 | 100% | Excellent | 3/Minimum discomfort 1 month after completion of therapy. Resolved in 1 week with nitroglycerin |
| 12/M/48 | 36 | No | 45 | Complete | 6/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 13/W/81 | NA | Topical corticosteroids, cryotherapy | 10 | Complete | 10/10 | 0/10 | 100% | Excellent | 6/Minimum discomfort 2 months after completion of therapy. Resolved in 10 days using nitroglycerin |
| 14/W/81 | 72 | Topical corticosteroids, cryotherapy, surgery | 67 | Complete | 7/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 15/M/41 | NA | Cryotherapy, surgery | 30 | None | 8/10 | 8/10 | 0% | Excellent | 0/Surgery owing to lack of response |
| 16/W/58 | 12 | Intralesional corticosteroids | 150 | Complete | 10/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 17/W/86 | 3 | No | 90 | Complete | 10/10 | 0/10 | 100% | Excellent | 4/No relapse |
| 18/W/83 | NA | No | 100 | Partial | 10/10 | 1/10 | 90% | Excellent | 4//No relapse |
| 19/W/72 | NA | No | 90 | Complete | 8/10 | 0/10 | 100% | Excellent | 6/Occasionally uses nitroglycerin because of minimum discomfort. Very good response |
| 20/W/62 | 48 | Topical corticosteroids, cryotherapy, excision | 60 | Partial | 10/10 | 2/10 | 80% | Excellent | 3/Minimum discomfort 3 months after completion of therapy. Resolved in 10 days with nitroglycerin |
| 21/M/73 | 72 | Surgery (2 operations) | 60 | Partial | 10/10 | 2/10 | 80% | Excellent | 12/No relapse |
| 22/M/73 | NA | No | 90 | Complete | 7/10 | 0/10 | 100% | Excellent (headache after using an excessive amount of the product, resolved when amount was reduced) | 7/No relapse |
| 23/M/75 | 3 | No | 60 | Complete | 7/10 | 0/10 | 100% | Excellent | 15/No relapse |
| 24/W/71 | 12 | No | 45 | Complete | 10/10 | 0/10 | 100% | Excellent | 4/No relapse |
| 25/W/72 | NA | No | 45 | Complete | 10/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 26/M/56 | 12 | No | 37 | Complete | 10/10 | 4/10 | 60% | Excellent | 4/No relapse |
| 27/W/80 | 240 | Surgery (2 operations) | 37 | Complete | 4/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 28/W/71 | 36 | Cryotherapy | 45 | Complete | 10/10 | 0/10 | 100% | Excellent | 3/No relapse |
| 29/W/62 | 4 | No | 90 | Partial | 8/10 | 2/10 | 75% | Excellent | 3/No relapse |
Abbreviation: NA, not available.
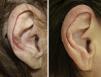
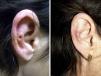
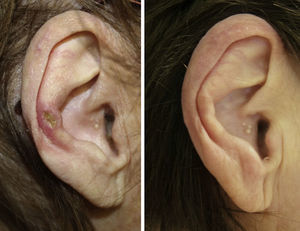
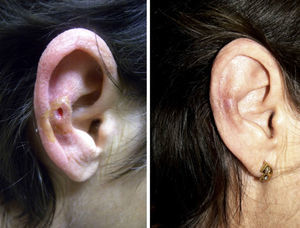
A clear clinical improvement was observed in 27 patients (93%), 17 of whom had a complete response (63%) and 10 a partial response (37%). Mean duration of treatment in the responders was 1.8 months (54.17 days) (Figures 1 and 2). Two patients (6.9%) had not responded (nonresponse) after 4 weeks of treatment and required additional treatment.
Tolerance was good in 28 cases (97%). Headache was recorded as a side effect of treatment in 1 case.
Responders were followed up after treatment for a mean period of 5.9 months (range, 3-18 months). After treatment, 48% of patients (14 patients) were followed up for more than 4 months. After 18 months of follow-up, only 1 patient had a clinical relapse, which occurred simultaneously with initiation of chemotherapy for breast cancer. Four patients reported episodic but mild discomfort in the area previously affected by CNH, with no apparent recurrence of symptoms. The discomfort was adequately controlled with occasional application of 0.2% topical nitroglycerin as needed. In 1 case, the lesion was treated surgically owing to insufficient response (improvement of 10/10 to 8/10) after 1 month of therapy.
DiscussionOur results show how CNH improved in most patients after treatment with 0.2% topical nitroglycerin with almost no side effects.
Several therapeutic options with varying efficacy are available, although few controlled studies in this area include both medical and surgical measures, such as topical corticosteroids,8 intralesional corticosteroids,9 cryosurgery,2 carbon dioxide laser treatment,10 and various surgical prodedures11,12 or even photodynamic therapy.3 Recently, 2% topical nitroglycerin was reported to be useful for the treatment of CNH.5–7The mechanisms that explain the pathogenesis of CNH and the response to nitroglycerin include underlying vascular disease, in which arterial flow is compromised by narrowing of the arterioles supplying the perichondrium. Specific physical factors, such as repeated injury, cardiovascular disease, or even autoimmune disease, can damage the vascular network of the perichondrium.13 In any case, involvement of the vascular network of the auricle, whether as a cause or consequence of local connective tissue degeneration, could account for the satisfactory results achieved treating CNH with topical nitroglycerin, which is a potent vasodilator.
The first report of the efficacy of topical nitroglycerin in CNH was by Flynn et al.,5 who treated 13 patients with 2% topical nitroglycerin. A clinical improvement was recorded in 12 patients. The response was complete in 61.5% and partial in 30.8%. Two patients (17%) experienced treatment-associated side effects in the form of dizziness and headache.5 Subsequently, Yelamos et al.6 reported the case of a woman aged 83 years who responded to 2% topical nitroglycerin, with no relapse 1 month after finishing treatment, which she tolerated well. Lastly, Garrido-Colmenero et al.7 reported a series of 11 patients treated with nitroglycerin patches (5mg) for 12hours per day for 2 months. A complete response was recorded in 63.3%. Two patients (18.1%) had moderate headache, leading treatment to be suspended, and 1 required surgery owing to an insufficient response.
To date, only the 2% topical nitroglycerin formulation (not the 0.2% formulation) has proven efficacious for the treatment of CNH. The 0.2% formulation has traditionally been used to treat other conditions such as anal fissure14 and is the one we recommended to the patients in the present study. In fact, based on published data, we initially contacted a compounding laboratory, where we were informed that it was not possible to prepare a 2% formulation of nitroglycerin. The active principle comes in the form of a powder (2%), which is mixed with excipient to make up the formula. When the solution is mixed, the concentration decreases until the desired limit is reached, although it is always lower than 2%. A formulation with a concentration of 2% or more could prove unstable and would have to be handled with care. Higuero15 recently reviewed the latest approaches in the management of anal fissures. In the section on topical nitrates, the author reported efficacy data for topical nitroglycerin concentrations ranging from 0.05% to 0.4%, although no mention was made of higher concentrations, which could lead to increased toxicity with no clear parallel increase in efficacy.15
The formulation can be obtained from most compounding laboratories. It is considered a conservative, nonaggressive, and inexpensive measure compared with other available options, which are usually successful but more aggressive, such as cartilage surgery that spares the overlying skin.11
The 0.2% concentration seems to be efficacious and universally well tolerated. In the series we report, only 1 of the 29 patients complained of headache after using an excessive amount of the product. This side effect resolved when the patient reduced the amount of product used to a standard thin layer.
Two patients did not respond and a third patient showed very little improvement (decrease in the pain scale from 10/10 to 8/10) after 4 weeks of treatment. All 3 reported having previously undergone surgery for diagnostic or therapeutic purposes. In addition, although most patients usually respond to treatment from the initial weeks of treatment, we do not know if prolonging treatment for more than 1 month could have led to a favorable outcome. It is also interesting that only 11.8% of complete responders had undergone surgery for treatment of the lesion and that this percentage rose to 70% in partial responders. These data could lead us to believe that previous surgical treatment of the lesion could produce a poorer response to topical nitroglycerin.
Our study is limited by its retrospective and descriptive design and by the lack of a control group in which patients were tested with the excipient (white petrolatum) with no active ingredient (0.2% topical nitroglycerin) or received another efficacious treatment in order to compare the responses.
In conclusion, 0.2% topical nitroglycerin is considered a conservative, efficacious, and safe option for the treatment of CNH that obviates surgical removal, which can sometimes be disfiguring.
Ethical DisclosuresProtection of Persons and AnimalsThe authors declare that this research did not involve experiments performed on humans or animals.
Confidentiality of DataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sanz-Motilva V, Martorell-Calatayud A, Gutiérrez García-Rodrigo C, Hueso-Gabriel L, García-Melgares ML, Pelufo-Enguix C, et al. La utilidad de la nitroglicerina tópica al 0,2% en la condrodermatitis nodular del hélix. Actas Dermosifiliogr. 2015;106:555–561.