Recent guideline on the management of urticaria recommends second-generation H1-antihistamine as the first-line therapy, with dose increases of up to fourfold if inadequately controlled. However, the treatment of chronic spontaneous urticaria (CSU) is often disappointing, so additional adjuvant therapies are needed to increase the effectiveness of first-line therapy, especially in patients who are refractory to the increase of antihistamine doses. Recent studies recommend various adjuvant therapy modalities for CSU, such as biological agents, immunosuppressants, leukotriene receptor antagonists, H2-antihistamine, sulfones, autologous serum therapy, phototherapy, vitamin D, antioxidants, and probiotics. This literature review was made to determine the effectiveness of various adjuvant therapies in managing CSU.
Las directrices actuales para el tratamiento de la urticaria crónica recomiendan los antihistamínicos H1 de segunda generación como tratamiento de primera línea, con un aumento de hasta 4 veces la dosis si no se controla. Sin embargo, la terapia en la urticaria crónica espontánea suele ser decepcionante, por lo que es necesario un tratamiento adyuvante adicional para mejorar la eficacia de la terapia, especialmente en los pacientes que son refractarios a dosis mayores de antihistamínicos. Las investigaciones más recientes recomiendan varias modalidades de tratamiento adyuvante para la urticaria crónica espontánea, como los agentes biológicos, los inmunosupresores, los antagonistas de los receptores de leucotrienos, los antihistamínicos H2, las sulfonas, la terapia con suero autólogo, la fototerapia, la vitamina D, los antioxidantes y los probióticos.
Esta revisión bibliográfica presenta estudios recientes sobre la eficacia de diversas terapias adyuvantes en el tratamiento de la urticaria crónica espontánea.
Nowadays, second generation of H1 antihistamine as the first line therapy is recommended by the recent management of urticaria guidelines with an increase in dosage 4 times higher when there is no improvement in the clinical symptoms.1 Despite the dosage enhancement, chronic spontaneous urticaria (CSU) therapy still requires a complement agent to maximize its effect, necessitating the use of adjuvant therapy to increase the effectiveness of the first line therapy. Adjuvant therapy for CSU is needed, especially for refractory patients receiving increased antihistamine doses. This literature review will discuss further a variety of adjuvant therapy and their role in the management of CSU. As shown in Table 1, the level of evidence (LoE) of the management is based on Oxford Centre for Evidence-Based Medicine 2011 for the therapeutic study and the simplified table of adjuvant therapy modalities can be seen in Table 2.
Level of evidence (LoE) Oxford Centre for Evidence-Based Medicine 2011 for therapy study.
| Level I | Level II | Level III | Level IV | Level V |
|---|---|---|---|---|
| Systematic review of randomized trials or n-of-1 trials | Randomized trial or observational study with dramatic effect | Non-randomized controlled cohort/follow-up study | Case-series, case control studies, or historically controlled studies | Mechanism-based reasoning |
Adjuvant therapy modality in the chronic urticaria treatment.
| Modality | Type | Indication | Dosage | Onset improvement | LoE |
|---|---|---|---|---|---|
| Biologic agent | Omalizumab | CSU/CindU | 150–300mg/4 week, sc | 1–2 weeks | I |
| Rituximab | CSU | 375mg/m2/week, iv | 1 week | IV | |
| Inhibitor TNF-α(etanercept) | CSU/CindU | 50mg/week, sc | 1 week | IV | |
| Immunosuppressant | Cyclosporin | CSU/CindU | 3–5mg/kgBB/day, po | 5–7 days | I |
| Methotrexate | CSU/CindU | 7.5–15mg/week, po | 3 weeks–months | II | |
| MMF | CSU | 1000–2000mg/day, po | 12–14 weeks | II | |
| LTRA | Montelukast | CSU/CindU | 10mg/day, po | 2–4 weeks | I |
| H2 antihistamine | Ranitidine | CSU | 150–300mg/day, po | 1–2 weeks | I |
| Sulfone | Dapsone | CSU/CindU | 50–100mg/day, po | 1–6 weeks | II |
| Sulfasalazine | CSU/CindU | 500–2000mg/day, po | 3–6 months | IV | |
| Phototherapy | NBUVB | CSU/CindU | 200mJ/cm2, 3×/week, naik 10–20% | 7–8 weeks | I |
| Autohemotherapy | AST | CSU | 0.05ml/kgBB/week, im | 4–7 weeks | I |
| Vitamin D | Vitamin D3 | CSU | 2000IU/day or 60,000IU/week, po | 4–12 weeks | I |
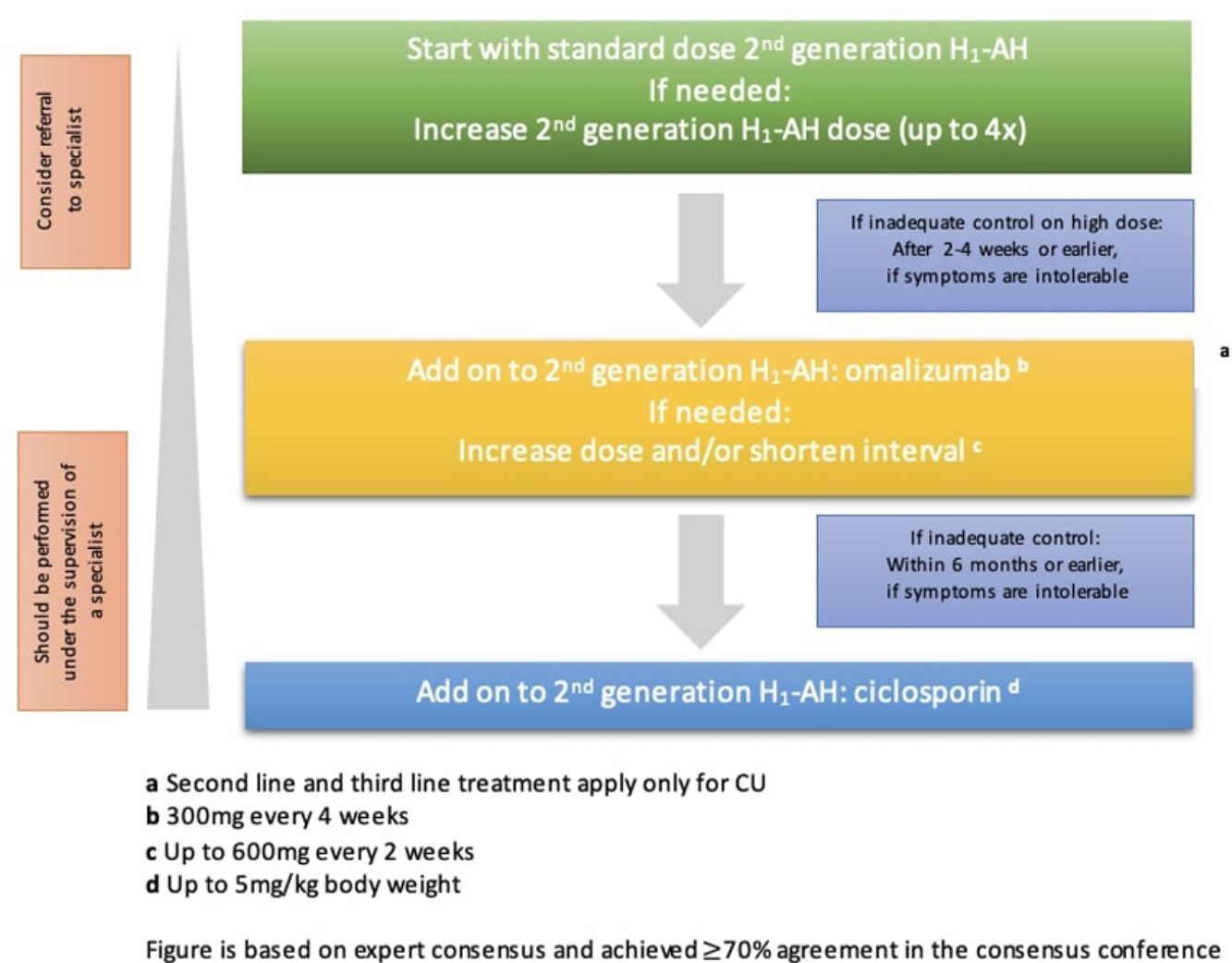
The goal of treating CSU is to resolve all symptoms through non-pharmacology approaches, including identification and elimination of the urticaria causes, avoiding trigger factors, and tolerance induction; then pharmacological therapy to prevent the release of mast cell mediators or the effect of mast cell mediators. The pharmacological treatment should allow the lowest dose to alleviate the symptoms. The treatment is adjustable, corresponding to the degree of disease activity.2 As illustrated in Fig. 1, EAACI/GA2LEN/EDF/WAO guideline in 2022 recommends the second generation of H1 antihistamine to be the first line of therapy for CSU, with the dosage enhancement up to 4 times higher if there is no improvement in the clinical symptoms.1
Treatment algorithm of CSU based on EAACI/GA2LEN/EDF/WAO in 2022. Available from: Zuberbier T, Abdul Latiff AH, Abuzakouk M, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2022;77:734–766.
Adjuvant therapy is an additional therapy that is given together with the first line therapy simultaneously with the purpose of increasing the therapy effectivity.3 Adjuvant therapy in CSU is needed primarily on patients with no response to the treatment or refractory towards increasing antihistamine doses. The following sections will discuss several adjuvant therapies and their roles in CSU.
Biologic agentBiological agent comes from living things and is used to prevent, diagnose, or treat a disease. The biologic agents used to treat chronic refractory urticaria are omalizumab, rituximab, intravenous immunoglobulin (IVIG), dan inhibitor TNF-α (infliximab, adalimumab, and etanercept); all used alongside the standard antihistamine regimen.4
OmalizumabOmalizumab (OMA) is a recombinant monoclonal antibody that comes from a human and acts as an anti-IgE antibody, preventing the binding of IgE to receptor cells.5,6 The effectiveness of OMA is proven and well tolerated in patients with refractory CSU. The EAACI/GA2LEN/EDF/WAO guidelines in 2022 recommend OMA as the third-line therapy for patients with chronic refractory urticaria towards the increase of H1 antihistamine dosage.2
The mechanism of action of OMA is not fully understood. Kaplan et al. have reported the mechanism of actions of OMA in the CSU treatment is by decreasing the free IgE and IgE receptors, reducing the mast cell ability to release histamine, restoring basopenia, restoring the IgE receptors function in basophile, reducing the activity of auto-antibody IgG towards IgE and its receptors, reducing the abnormal IgE intrinsic activity, reducing the auto-antibody IgE towards an antigen or an unknown auto-antigen, and reducing abnormal coagulation which related to the disease activity through in vitro.7
The effectivity of the OMA addition in the CSU treatment was reported in many phases II8,9 and phase III10–12 clinical trials, as well as meta-analysis.13 The three phase III clinical trials observed CSU/CindU moderate–severe patients who did not respond to the standard treatment and other additional therapies were given OMA 75mg, 150mg, or 300mg for 12–24 weeks, and there was a decrease in the itch severity score (ISS) on the 12-week treatment. Casale et al.14 have reported that there were more patients with urticaria activity score 7 (UAS7) ≤6 (symptoms are well controlled) or UAS7 0 (symptoms are perfectly controlled) when treated with OMA 300mg compared to placebo.
OMA is effective in the dosage of 150–300mg per month. The dosage and duration of the therapy may vary between countries. Generally, OMA is well tolerated, and the most common side effects are nasopharyngitis, sinusitis, URTI, headache, and cough.14 The risk of anaphylactic may occur 2h after the first administration of OMA; thus, careful observation is needed.14
RituximabRituximab is a chimeric monoclonal antibody to CD20. The mechanism of action of rituximab in CSU prevents the production of autoantibody.15 The rituximab treatment result varies widely. A case report of a patient with pressure urticaria who failed in the first and second line of therapy had received rituximab 375mg/m2/week infusion 4 times; however, there was no improvement. A case report of CSU and immunodeficiency treated by rituximab 375mg/m2/week, 4 times, showed a perfect recovery of urticaria symptoms within 1 week, with remission of more than 1 year, and later the symptoms will be easily treated by an antihistamine.16 Another case report of refractory autoimmune CSU was given rituximab per week (4 times in dosage, within 4 weeks) along with the administration of methotrexate showed a perfect remission 6 weeks after the last administration.17 There was no randomized controlled trials (RCT) study of rituximab in CSU.
Inhibitor TNF-αSome TNF-α inhibitors drugs such as etanercept, infliximab, and adalimumab were used to treat CSU or vasculitis urticaria based on the postulation that TNF may have important roles in some types of urticaria.18,19 For example, etanercept 2×25mg/week is useful to treat delayed pressure urticaria and psoriasis. On the 5-day of treatment, the urticaria was resolved and did not appear until the end of the therapy.20 A case series of 6 patients with chronic idiopathic urticaria or vasculitis urticaria treated by inhibitor TNF-α showed a dramatic clinical improvement in all patients.21
Serious infections such as tuberculosis, fungal infection, lymphoma, and malignancy were reported. This drug is not recommended for CSU treatment due to its lack of evidence to support the safety and efficacy of the treatment. Furthermore, there is no clinical trial comparing this drug to OMA, in which the safety and efficacy have been tested.
ImmunosuppressantCyclosporinA low dose of cyclosporin is commonly used in severe and refractory CSU/CindU patients. The EAACI/GA2LEN/EDF/WAO guidelines in 2022 recommend the use of cyclosporin as the fourth line therapy in a patient with CSU resistant to H1 antihistamine and OMA. Cyclosporin acts as an immunosuppressant which effect is known to attenuate T cell activity. In CSU, cyclosporin has a role in some mechanisms, such as calcineurin inhibitors, which hinder the calcium-dependent release by histamine, C4 leukotriene, and other mast cell mediators and several other cells. Cyclosporin also disrupts the TNF-α activity and secondarily inhibits neutrophile accumulation.22
A systematic review in 2018 showed a significant improvement in urticaria symptoms in CSU patients who were given a relatively high dose cyclosporin (5–6mg/kg/day). However, the patient usually stops the therapy due to the occurrence of side effects. The majority of new studies use a lower dose (2–4mg/kg/day) or an initial therapy with a high dose to continue tapering down the dose to get the lowest effective dose.22 Studies done in the paediatric population also use a low range of doses. Randomized clinical trials involving CSU refractory patients resistant to standard antihistamine compared a group receiving 4mg/kg/day cyclosporin and a group receiving a placebo for 4 weeks. Eight out of 19 (42%) patients receiving cyclosporin showed an improvement compared to placebo. Mild side effects were observable in 29 out of 30 subjects.23
There is still no guideline for the optimal cyclosporin dose in CSU. The last systematic review recommends the initial therapy with 3mg/kg cyclosporin, divided into 2 doses. The majority of adult patients were given the dosage of 100–150mg, 2 times per day. Some of the patients showed improvement in 1–2 weeks, whilst most other patients showed improvement within 3 months.22 As for the mild side effects of cyclosporin use (dosage related) are paresthesia, gastrointestinal symptoms, and headache. The dosage reduction may subside the symptoms. Hypertension and kidney insufficiency is a severe and uncommon side effect indicating the termination of the therapy.22
MethotrexateSeveral case reports and case series have reported the effectiveness of methotrexate in reducing the symptoms of CSU patients with steroid dependence24,25 and vasculitis urticaria.26 An RCT in India concluded that the addition of methotrexate (7.5–15mg per week) for 3 months in chronic refractory urticaria did not show any significant benefit.27 Although available data is still limited, several studies recommend methotrexate as the alternative therapy in some refractory urticaria cases, mainly due to its affordable price, availability, schedule-friendly, and wide acceptance.
Mycophenolate mofetilMycophenolate mofetil (MMF) is an immunomodulator agent to treat solid-organ transplant rejection and several dermatosis conditions (off-label). The mechanism of MMF immunomodulator in chronic autoimmune and idiopathic urticaria is not fully known. MMF is known to be effective in the urticaria treatment by inhibiting the autoantibody production towards high affinity of IgE receptors and/or IgE, as well as reducing the adhesion molecule expression to endothelial cells, thus inhibiting the leucocyte invasion to the skin.28
In a clinical study of 9 refractory CSU patients resistant to antihistamine and/or steroids, the administration of 2×1000mg MMF for 12 weeks reduced the UAS score and curbed the disease activity even without steroids.29 A retrospective study of 19 patients with autoimmune urticaria and chronic idiopathic urticaria showed 89% controlled urticaria symptoms for 14 weeks of MMF consumption (dosed at 1000–6000mg/day, in divided dosage). However, it was reported that the most common side effects are gastrointestinal symptoms.30 Nevertheless, due to lack of scientific evidence, doubtful effect, high price, and side effects reported, MMF is not recommended to be used as a treatment in the guideline for CSU/CindU patients.
Leukotriene receptor antagonists (LTRA)In most, CSU treatment is hard to be controlled only with antihistamines. Therefore, a suspicion of other mediators which have a role other than histamines, such as kinin, prostaglandin, and leukotriene, which may be responsible for some of the urticaria symptoms which cannot be controlled with antihistamine. Cysteinyl leukotrienes is a potent pro-inflammatory mediator which can be inhibited by LTRA like montelukast, zafirlukast, and pranlukast.31 The use of these drugs in asthma and allergy rhinitis is already proven beneficial. CSU guidelines by the British Society for Allergy and Clinical Immunology (BSACI) and The American Consensus Document recommends the addition of LTRA before prescribing OMA and cyclosporin.
A systematic review by De Silva et al.31 suggested a reduction in the urticaria lesion number in patient with LTRA monotherapy compared to placebo. The combination of antihistamine and LTRA is proven to be beneficial in many studies even though there was one study that showed contradictive findings. Generally, some of the most recent studies supported LTRA in combination with antihistamine, and the effect of additional LTRA in patients with antihistamine therapy showed beneficial results when prescribed 10mg/day for 2–4 weeks. One of the weaknesses of LTRA therapy is the price that is more expensive for one month of therapy compared to the antihistamine within the same time range. However, as a combination therapy with antihistamine, LTRA showed a good tolerance with minimal side effect.31
Antihistamin H2H2 antihistamine is a class of drugs binding to H2 histamine receptors which are commonly found in stomach cells. H2 antihistamine is clinically used in acid-related gastrointestinal conditions therapy such as peptic ulcer, gastroesophageal reflux (GERD), and dyspepsia. H2 antihistamine is also used in urticaria therapy; generally, it is combined with H1 antihistamine. Several drugs in this class are cimetidine, ranitidine, famotidine, roxatidine, lafutidine, and nizatidine.
A systematic review concluded that the combination of H1 antihistamine and H2 antihistamine in a patient with CSU showed a clinical improvement compared to H1 antihistamine alone, even though the level of evidence is still minimal.32 The synergism between H1 antihistamine and H2 antihistamine is still debatable; however, the possible cause may relate to its pharmacokinetic effect in which H2 antihistamine caused the level of H1 antihistamine in the plasma to increase. An RCT in 45 CSU patients receiving terfenadine and ranitidine as an adjuvant showed a better result compared to the administration of terfenadine only, mostly to reduce the itchy complaint; however, there was no significant effect on the urticaria symptoms.33
Majority RCT and some case reports showed that adding H2 antihistamine still provides insufficient benefits; moreover, it did not meet the expectations of some studies.32 Therefore, this drug is not included in the primary treatment of CSU based on EAACI/GA2LEN/EDF/WAO guidelines in 2022. Generally, H2 antihistamine can be well tolerated. Some side effects reported were hypotension, headache, dizziness, diarrhoea, rash, gynecomastia, loss of libido, and impotency.
SulfoneDapsone and sulfasalazine have been used in several studies as adjunctive therapy for CSU cases.34
DapsoneDapsone worked by suppressing prostaglandin and leukotriene activity, influencing the release or function of neutrophile lysosomal enzymes,35 interfering with integrin-mediated neutrophil adhesion, inhibition of neutrophil recruitment, and signal activation,36 as well as removing oxygen-free radical intermediates.37
A RCT study consisting of 22 CSU patients treated with dapsone 100mg/day for 6 weeks has been shown to have good results in controlling urticaria and itching symptoms.38 A RCT reported that the combination of dapsone with antihistamines compared to single antihistamine use shown to have a decrease in UAS scores with complete remission in some cases.39 Furthermore, dapsone is effective in treating urticaria vasculitis and idiopathic angioedema.40–42
Side effects of dapsone include methemoglobinemia, peripheral neuropathy, and hepatotoxicity. Therefore it is necessary to check for G6PD deficiency before starting treatment. Due to the limited availability of scientific evidence and the possibility of serious side effects, the EAACI/GA2LEN/EDF/WAO 2022 guidelines do not recommend the use of dapsone as therapy for CSU/CindU.34
Sulfasalazine (SSZ)The mechanism of action of SSZ in CSU patients includes the release of adenosine, reduced synthesis of leukotrienes and prostaglandins, inhibition of IgE-mediated mast cell degranulation, also inhibition of early-phase proliferation and differentiation of B lymphocytes.43
Studies have concluded that sulfasalazine is useful as an adjunct to standard therapy for patients with refractory symptoms.43,44 A retrospective study of 39 patients with refractory CSU towards antihistamines and other therapies were given sulfasalazine as an adjunct therapy, with an initial dose of 500mg per day, then increased per week up to 2000mg per day (and up to 3000mg per day in 15 patients) if tolerated.43 84% of patients had improvement within 3 months, with a mean duration of therapy of 74 weeks.
In adults, sulfasalazine therapy can be started at a dose of 500mg, 1–2 times per day, and increased gradually to 1g, twice daily. The optimal duration of therapy varies from individual to individual. In general, sulfasalazine is well tolerated in the majority of patients. Side effects include nausea, headache, mild and transient leukopenia, and to a lesser degree agranulocytosis.
PhototherapyPhototherapy using psoralen with ultraviolet A(PUVA) or narrow band UVB (NVUVB) and UVA is beneficial in CSU.45,46 Phototherapy is also considered a treatment option in patients with intolerance to systemic drugs. The skin directly exposed to ultraviolet radiation experienced dramatic improvement; this is the basis for the assumption that there are mediators and local cells that act as primary targets.47 Although the effectiveness of NBUVB in CSU has been suggested in several studies, the exact mechanism of NBUVB in CSU is still unclear. NBUVB has a suppressive effect on systemic immune response and natural killer (NK) cell activity, lymphocyte proliferation, and regulation of cytokine production by TH1 (IL-2, IFN-g) and Th2 (IL-10). NBUVB also has an inhibitory effect on proinflammatory mediators and cytokines.
A systematic review concluded that NBUVB is an adjuvant therapy modality in the management of refractory CSU.48 A RCT of 50 CSU patients refractory to H1 antihistamines and oral steroid-dependent patients compared the administration of NBUV and PUVA. There was greater clinical improvement in the group with NBUVB than in the PUVA group. Side effects only occur in a small number of patients, including tanning and xerosis.49
A study by Sheikh et al.50 shows that NBUVB can be an effective adjuvant therapy with antihistamines in patients with CSU. The combination allows a greater reduction in UAS than single antihistamine use. In this study, the initial dose of phototherapy started at 200mJ/cm2 in 16 sessions over 8 weeks. Berroeta et al.51 reported that the median of phototherapy sessions in CSU patients was 22 sessions, the frequency being 3×/week with an initial dose of 70%, starting with the minimum erythema dose then increased by 10–20% per visit, and a media dose of 1238mJ/cm2 (range 100–2111mJ/cm2). Meanwhile, Engin et al.52 suggested that the number of therapy was 20 sessions, with a frequency of 3×/week and an initial dose of 200mJ/cm2, increased by 10–20% to a maximum dose of 1300mJ/cm2. The side effects of NBUVB therapy include erythema, pruritus, and vesiculation.50
Autologue serum therapyApproximately 30–50% of patients with CSU have autoantibodies binding to the high-affinity IgE receptor FcɛRiα on basophils or mast cells that produce histamine and IgE.53 Hide et al.,54 reported that intracutaneous injection of serum, the autologous serum skin test (ASST), causes a type of rapid hypersensitivity reaction in patients with CSU, which is referred to as autoreactive or autoimmune CSU. These patients had scores of itching or urticaria and more severe systemic symptoms associated with other autoimmune diseases. Because circulating histamine-releasing factors play a role in the induction of urticarial symptoms in ASST-positive CSU patients, autohemotherapy is considered promising and has potential as a treatment option in chronic autoimmune urticaria.
A systematic review by Chang et al.55 in 2019 concluded that autologous whole blood (AWB) and autologous serum therapy (AST) were not significantly more effective in relieving CSU symptoms than placebo. Nageswaramma et al.56 reported 50 CSU patients with positive and negative ASST were given autologous serum therapy (AST) injections weekly for 9 weeks and followed for 12 weeks after 9 weeks of injections. Urticaria symptoms decreased at week 4, and antihistamine use decreased 100% from baseline in both groups. The patient was found to be in complete remission at week 21.
In a study by Kumaravel et al.,57 involving 200 CSU patients, AST was intramuscularly administered to 47 ASST (+) patients every week for 9 weeks and followed for 3 months. At the end of therapy, none of the patients had severe TSS, 9 patients were symptom-free, and the majority had only mild TSS. As reported by Karn et al.,58 102 patients with CSU patients with both ASST (+) and (−), an intramuscular injection of 0.05ml/kg autologous serum was administered weekly for 10 weeks. There was a significant improvement in UAS at week 10 compared to baseline in both groups. In patients with chronic autoimmune urticaria, AST is an inexpensive, effective therapeutic modality with minimal side effects.
Vitamin DVitamin D plays an important role in the innate and adaptive immune systems through stimulation of Toll-like receptors, increasing production of pro-inflammatory cytokines, and possibly enhancing the T helper 2 response. This mechanism may explain the association of vitamin D with several autoimmune allergic diseases, including CSU.4
A systematic review by Tuchinda et al.32 stated that high-dose vitamin D supplementation could significantly reduce CSU activity. Another study stated that vitamin D supplementation of 2000IU/day and 60,000IU/week reduced disease activity in almost all CSU patients. Of the various regiments, higher doses of vitamin D (vitamin D3 at least 28,000IU/week for 4–12 weeks or vitamin D2 140,000IU/week for 6 weeks) were reported to be more effective. Although studies are relatively sparse, CSU patients with low serum vitamin D at study entry tend to improve with high-dose vitamin D supplementation. Vitamin D has a high safety dose limit. The maximum tolerable intake is 4000–10,000IU/day for adults and the elderly but lower for infants and children.
Although there are no reports of side effects during administration of vitamin D therapy, patient safety should be considered when using high doses of vitamin D. Assessment of serum vitamin D levels can be used to evaluate the safety and determine the relationship to therapeutic outcomes, and caution should be exercised regarding potential side effects when serum 25(OH)D levels are more than 50ng/ml (125nmol/l). In recalcitrant CSU patients with low serum vitamin D levels, high-dose vitamin D supplementation for 4–12 weeks can be used as adjuvant therapy.
ConclusionOmalizumab become the first adjuvant therapy option, however in some countries omalizumab is still not available and accessible. Other reasons are cost-effectiveness (omalizumab is high in price) and not covered by government health insurance. Patients need to be assessed comprehensively prior choosing the treatment. Phototherapy is one of the alternative treatment considering its mild side effects, however it shows lack in patient adherence, therefore systemic therapy is recommended such as methotrexate and other agents.
Several adjuvant therapy modalities in CSU can act synergically to increase the effectivity of the first line therapy, which is H1 antihistamine second generation in CSU treatment. Further investigation is required to evaluate adjuvant therapy modalities with a better and more consistent method so, it can improve the clinical practice.
Conflict of interestThe authors declare that they have no conflict of interest.