The telocytes (TCs) are novel interstitial cells that have been overlooked for a long time due to their histologic similarity to other stromal cells. TCs can be separated from the stromal cells based on their distinct immunohistochemical, ultrastructural, and molecular features. Functionally, TCs are involved in the tissue renewal, mechanical support, and immune modulation. These cells are also involved in the signal transduction either through their direct interactions with the neighboring cells or through the paracrine signaling via extracellular vesicles. TCs are damaged in several inflammatory and fibrotic conditions such as ulcerative colitis, Crohn's disease, hepatic fibrosis, psoriasis, and systemic sclerosis. The transplantation of TCs in the damaged tissue can promote tissue regeneration. Therefore, enhancing tissue TCs either by their transplantation or by promoting their survival and growth using novel medications represents novel therapeutic strategy in the future. In this review, we addressed several aspects of TCs including their origin, distribution, morphologic features, and functions. We also discussed their involvement of the cutaneous TCs in the development various pathologic conditions.
Los telocitos (TC) son células intersticiales noveles que han sido subvaloradas durante mucho tiempo debido a su similitud histológica con otras células estromales. Los TC pueden separarse de las células estromales debido a sus distintas características inmunohistoquímicas, ultraestructurales y moleculares. A nivel funcional, los TC están implicados en la renovación tisular, el soporte mecánico y la modulación inmune. Dichas células están implicadas también en la transducción de señal, bien mediante sus interacciones directas con las células circundantes, o bien mediante la señalización paracrina, a través de las vesículas extracelulares. Los TC se ven dañados en ciertas situaciones inflamatorias y fibróticas tales como colitis ulcerosa, enfermedad de Crohn, fibrosis hepática, psoriasis y esclerosis sistémica. El trasplante de TC en el tejido dañado puede promover la regeneración tisular. Por tanto, mejorar los TC tisulares mediante trasplante o promoción de su supervivencia y crecimiento, utilizando medicaciones novedosas, representa una estrategia terapéutica innovadora para el futuro. En esta revisión abordamos diversos aspectos de los TC, incluyendo su origen, su distribución, sus características morfológicas y sus funciones. También tratamos la implicación de los TC cutáneos en el desarrollo de diversas situaciones patológicas.
It was in 1911 when Santiago Ramón y Cajal identified a new type of cells with long cytoplasmic processes within the muscle layer of the human alimentary canal. Cajal coined these cells as “interstitial neurons” due to their characteristic cytoplasmic projections and location among the smooth muscle cells and the nerve endings.1 In 1977, Faussone-Pellegrini et al. investigated the ultrastructural features of these interstitial neurons using electron microscopy. They indicated that these cells were not true neurons, and therefore they re-named them as ‘interstitial cells of Cajal’ (ICCs). The authors described the ultrastructural features of ICCs as spindle-shaped cells with long cytoplasmic processes by which these cells can interact with each other and with other cell types.2 In 1996, Lecoin et al. confirmed the mesenchymal origin of ICCs. They also indicated that ICCs in chick embryos express the gene encoding the cytokine receptor tyrosine kinase “Kit”.3
In 2005, Popescu et al. described cells that closely resemble ICCs in the exocrine pancreas, and they coined those “interstitial Cajal-like cells (ICLCs).4 In 2010, Faussone-Pellegrini and Popescu indicated that these cells have unique physical properties, such as the presence of oval nuclei surrounded by scanty cytoplasm and telopodes (Tps). They coined these cells “telocytes (TCs)”. They indicated that TCs are entirely different from ICCs because they have unique ultrastructural, immunohistochemical, and genetic features, as well as other protein expression profile.5
The Distribution of TCs In the Human TissuesTCs have been detected in the tissue of several vertebrates such as mice, rats, guinea pigs, chickens, and humans. Initially, TCs were thought to be predominantly located in the gut of the human body.6 However, growing evidence indicates that TCs are present in almost all human organs such as the heart,7 blood vessels,8 lungs,9 meninges,10 skin,10,11 salivary glands,12 liver, gall bladder,13 pancreas,4,14,15 bone marrow,16 breast,15 fallopian tube,17 placenta,18 myometrium,14 kidney, urinary bladder, and prostate.19 In these organs, TCs are commonly located in the interstitial tissue, where they can interact with the stromal cells, immune cells, and the surrounding blood vessels. The distribution of the TCs in different human tissues and their cellular interactions is summarized in Table 1.
The Distribution of the Telocytes in the Different Human Tissues and Their Cellular Interactions With Other Cells.
| Organ | Localization | Associated cells | References |
|---|---|---|---|
| Heart | Endocardium, myocardium, and epicardium | Capillaries, nerves, lymphocytes, plasma, and satellite cells | 7 |
| Heart valves | Apex and base of all heart valves | Stem cells | 58 |
| Blood vessels | The surrounding muscle layer | Arterioles, capillaries, and venules | 8 |
| Lungs | Subepithelial stroma and bronchoalveolar junctions | Epithelial and stem cells | 9 |
| Meninges and choroid plexus | Interstitium | Blood vessels, ependymal, and stem cells | 10 |
| Skeletal muscle | Interstitium | capillaries, nerves, and the myocytes | 9 |
| Dermis | In the papillary and reticular dermis, around sebaceous and eccrine sweat glands, and perifollicular sheath | Immune and stem cells, blood vessels, and arrector pili muscles | 11 |
| Limbus and uvea | Conjuctival lamina propria, sclera, iris stroma, pars palana of ciliary body, and subcorneal epithelium | Epithelial and stromal stem cells, melanocytes, macrophages, and nerve fibers | 59 |
| Esophagus | Lamina propria and muscle layer | Lymphocytes, capillaries, and nerves | 60 |
| Duodenum | In lamina propria and surrounding the crypts | Immune cells, nerves, and blood vessels | 6 |
| Jejunum | Lamina propria and muscularis mucosae | Immune cells, nerve fibers, epithelial, and smooth muscle cells | 61 |
| Colon | Lamina propria | Muscle cells, nerve fibers, blood vessels, and epithelial stem cells | 6 |
| Parotid gland | Interacinar and subductal stroma | Acini, ducts, and blood vessels | 12 |
| Gall bladder | Sub epithelium and between smooth muscle fibers | Capillaries and smooth muscle cells, and nerve bundles | 13 |
| Pancreas | Exocrine pancreas | Acinar and ductal cells, and blood vessels | 4 |
| Bone marrow | Bone marrow | Capillaries and arterioles | 16 |
| Mammary gland | Stroma | Nerve fibers, immune cells, fibroblasts, and capillaries | 15 |
| Fallopian tube | Lamina propria and between smooth muscle fibers | Epithelial cells and capillaries | 17 |
| Myometrium | Between muscle fibers | Smooth muscle cells, nerves, and capillaries | 14 |
| Placenta | Connective tissue core of the villi | Vascular smooth muscle cells and collagen fibers | 18 |
| Kidney | Sub-capsular space | Macrophages | 19 |
| Urinary bladder | Smooth muscle bundles of muscularis mucosa | Nerve bundles, capillaries, and smooth muscle cells | 19 |
| Prostate | Stroma | Blood vessels, immune cells, and nerve bundles | 62 |
The two-dimensional (2D) transmission electron microscopy (TEM), focused ion beam-scanning electron microscopy (FIB-SEM) tomography, and the three-dimensional (3D) imaging techniques are used to examine the ultrastructural features of TCs.20–24 TCs have cell bodies and cytoplasmic processes (Tps) with unique ultrastructural features that distinguish them from other stromal cells (Table 2). Several factors can affect the density and the morphology of TCs, such as pregnancy, aging, and oxidative stress. For instance, during pregnancy, the TCs density of the endometrium significantly increases, whereas the myometrial TCs density significantly decreases as compared to the non-pregnant uteri.23 Alternatively, oxidative stress and aging affect the morphology of TCs with the formation and migration of Tps of TCs.17,24–26
The Ultrastructural Features of the Telocytes.
| Features | Description | References |
|---|---|---|
| Location | -Non-epithelial spaces | 17,20,22–26 |
| Cellular contacts | -Epithelial cells, nerve fibers, capillaries, and smooth muscle cells | |
| Cell body | -Small cell body that measures about 9–15μm.-The shape of the cellular body differs according to the number of Tps present, so the shape may be pyriform or spindle or triangular with 1 or 2 or 3 Tps respectively. If ≥3 Tps, the body may be stellate | |
| Nucleus | -Single, oval-shaped nucleus with condensed chromatin (40–45% euchromatin, and 55–60% heterochromatin)-No obvious nucleolus | |
| Cytoplasm | -Scanty cytoplasm that contains few organelles such as mitochondria, small Golgi apparatus, endoplasmic reticulum, microtubules, intermediate, and thin filaments-Several caveolae are detected on the cell membrane | |
| Cytoplasmic processes (Tps) | -Number: 1–5, average of 2–3 in the two-dimensional sections-Length: 10–1000μm-Thickness: 0.05–0.2μm-Branching: dichotomous branching pattern-Shape: alternating long thin segments called “podomers” (75–80nm) and dilated segments called “podoms” (250–300nm) creating a bead on a string pattern-Podoms contain the functional units needed for Ca2+ uptake/release, including mitochondria, endoplasmic reticulum, and caveolae-Podomers are anchored by homocellular and heterocellular junctions (allow interactions with the nerve bundles, blood capillaries, smooth muscle fibers, stem cells, and the extracellular matrix such as collagen and elastin fibers) |
In the hematoxylin and eosin stained sections, it is hard to distinguish TCs from fibroblast-like stromal cells. The thickness of histological sections hinders capturing the entire 3D structures of the TCs and detecting the distribution of the Tps of these cells.27 The immunohistochemistry combined with TEM represents a reliable method of identifying TCs. Variable immunohistochemical stains are used to label TCs in the human tissues as C-Kit/CD117, platelet-derived growth factor receptor α (PDGFR α), CD34, α-smooth muscle actin (α-SMA), and vimentin. However, none of these stains is specific by itself for the detection of TCs. Therefore double immunostaining methods such as CD34/vimentin, CD34/PDGFRα, or CD34/c-kit are more reliable for TCs detection.24,28 A summary of the immunostains used to highlight TCs is presented in Table 3.
The Immunohistochemical Features of the TCs in the Different Human Tissues.
| Organs | Immune profile of the telocytes | References |
|---|---|---|
| Heart | CD34, c-kit, and S100 | 7 |
| Heart valves | CD34, c-kit, vimentin, and PDGFR-β | 58 |
| Lungs and trachea | CD34, c-kit, vimentin, PDGFR- β, Sca-1, and VEGF | 9,29 |
| Meninges and choroid plexus | C-Kit | 10 |
| Gastrointestinal tract(lamina propria) | CD34, c-kit, vimentin,PDGFRα, FOXL1,GLI1, SOX6, and CD90 | 32,63,64 |
| Gall bladder | CD34, c-kit, and vimentin | 13 |
| Salivary glands | c-kit, vimentin, and α-SMA | 12,65 |
| Pancreas | CD34, c-kit, vimentin, occasional α-SMA, and S100 | 65 |
| Myometrium | CD34 and c-kit | 66 |
| Placenta | CD34, c-kit, and vimentin | 18 |
| Fallopian tube | CD34, c-kit, S100, and occasionally vimentin | 17 |
| Ureter and urinary bladder | Double positivity for CD34/calreticulin, and PDGFRα/calreticulin | 67 |
| Kidney | CD34, c-kit, and vimentin | 68 |
| Skin | CD34, c-kit, and vimentin | 11 |
| Striated muscle | c-kit, PDGFR- β, vimentin, caveolin-1, and VEGF | 29 |
There are also organ-specific subtypes of TCs, which display immunoreactivity for additional immunostains. For instance, TCs in the lung also express stem cell markers such as stem cell antigen-1 (Sca-1),9,29 suggesting their role in tissue regeneration. TCs in the skeletal muscle express VEGF indicating their role in angiogenesis.29 TCs from myometrium.30 Fallopian tubes,30 lamina propria of the renal pelvis, ureter, bladder, and urethra31 express estrogen and progesterone steroid receptors indicating that these cells act as sensors for steroid hormones and modulate signal transduction through steroid receptors. In the intestine, TCs express Foxl1 protein that is required for stem cell maintenance.32 In the spleen, the TCs express Nanog (a transcription factor involved in the self-renewal of undifferentiated embryonic stem cells) and Sca-1.33 The cardiac TCs express CD34/c-kit, CD34/vimentin, and CD34/PDGFR-β positive.34,35
The Genes, Proteins, and MicroRNAs of the TCsSong et al. examined the gene expression profiles (chromosomes 1, 2, 3, 17, and 18) in TCs of the mouse lung tissue.36 They reported the upregulation of several genes in TCs such as collagen type IV, connective tissue growth factor, nidogen1, matrix metallopeptidases 3 and10, tissue inhibitor of metalloproteinase-3, and transgelinas as compared to the stromal cells. These genes have regulatory effects on cell signaling, division, migration, adhesion, embryogenesis, and tissue repair. They also have essential roles in tissue homeostasis, immune modulation, and maintenance of the oxidative microenvironment. Accordingly, they can prevent tumorigenesis and anti-inflammatory responses.36 The TCs also express many pro-angiogenic microRNAs (miR126, miR-21, miR130a, miR-143, miR-503, miR-27b, miR-199a, and miR-100).37,38
The Functions of the TCs in Human biologyTCs have several functions, as summarized in Table 4, including (i) cell-to-cell communication and signaling, (ii) mechanical support and organ structure, (iii) tissue repair, angiogenesis, regeneration, and homeostasis, (iv) immune modulation and surveillance, and (v) hormone sensors in the female reproductive tract.
Functions of Telocytes in Human Biology.
| Function | Mechanism and effect | References |
|---|---|---|
| Cell-to-cell communication and signaling with homo- or heterogenous neighboring cells | The signaling is established by the gap junctions which facilitate direct contact among the cells and allow the passage of ions and molecules (e.g., proteins or microRNA) among them | 24 |
| Mechanical support of the different tissues | The distinct 3D structure of TCs and their ability to connect the surrounding cells provide structural support throughout the different tissues. For example, TCs can establish mechanical support to protect against bladder wall deformation during distension and relaxation | 67 |
| Tissue repair, angiogenesis, regeneration, and homeostasis | These roles are mediated by two mechanisms: | |
| -First: following tissue damage, the TCs act as CD34-positive progenitor cells that become activated with their subsequent proliferation, alterations in their morphology, distribution, and the differentiation into other cell types. | 69,70 | |
| -Second: the TCs act as interstitial cells within stem cell niches (microenvironment for stem cells) where they are integrated with stem cells and other components of the niche such as extracellular matrix and blood vessels. The TCs in the niches carry out several functions including the homeostatic control, support, nurse, signal induction, and regulation | 71,72 | |
| Immune modulation and surveillance | -The TCs can activate several immune cells (lymphocytes, mast cells, macrophages and eosinophils) by secretion of several cytokines such as IL-10, IL1-R1, TNFα, and IL-6, and therefore, they play some roles in immune regulation | 73 |
| -The TCs constitute a functional component of the main immunologic barriers in the human tissues as the blood-testis barrier and blood-myocardium barrier | 74 | |
| Hormone sensors in the female reproductive tract | TCs can express estrogen and progesterone receptors and they might regulate myometrial contractions and fallopian tube motility by gap junctions or juxtacrine and/or paracrine signaling | 30 |
Alterations of the TCs occur in several diseases and are collectively known as “telocytopathies”. A summary of these conditions is presented in Table 5. There is significant functional disturbance and loss of TCs in several chronic inflammatory and fibrotic diseases. These include scleroderma, primary Sjögren's syndrome, ulcerative colitis, Crohn's disease, and liver fibrosis. It is unclear whether this deranged function and quantitative loss are the primary cause of disease development or occur as a result of other unknown factors that disrupt the cellular microenvironment with subsequent TCs loss.39 Some tumors arise from TCs (telocytomas), such as gastrointestinal stromal tumors (GISTs), inflammatory fibroid polyps of the gastrointestinal tract, and extra-gastrointestinal stromal tumors.40 These neoplasms bear PDGFRα mutation, one of the specific antigens of TCs in the gastrointestinal tract.41 Some extra-gastrointestinal stromal tumors (eGISTs) in the prostate,42 uterus,43 and vagina44 arise from the TCs because these tumors and TCs share the expression of c-kit (CD117) protein.6 In 2018, Ricci et al. suggested the term “telocytoma” for tumors with possible telocytic origin.40 In lobular breast carcinoma, there is a proliferative effect of TCs by secreting extracellular vesicles suggesting that TCs have been activated at the wrong time and place.45
The Pathological Conditions (Telopathies) Associated With the Dystrophy (Altered Functions) and Decreased Density of the Telocytes.
| Organs | Diseases (telocytopathies) | References |
|---|---|---|
| Skin | Squamous cell carcinoma, basal cell carcinoma, psoriasis, and systemic sclerosis | 51,5247,49,50 |
| Intestine | Inflammatory bowel diseases (ulcerative colitis, and Crohn's disease) | 75,76 |
| Salivary glands | Sjögren's disease | 77 |
| Gastric antrum | Inflammatory fibroid polyp | 43 |
| Gallbladder | Gallstones | 78 |
| Pancreas | Extra-gastrointestinal stromal tumor. | 79 |
| Kidney | Uretero-pelvic junction obstruction. | 80 |
| Urinary bladder | Neurogenic detrusor over-activity | 81 |
| Lung | Fibrosis after pneumonia | 82 |
| Testis | Hyperplasia of the Leydig cells in undescended testes(cryptorchidism) | 83 |
| Prostate gland | Prostate cancer and benign prostate hyperplasia | 84 |
| Uterus | Leiomyomas | 85 |
| Fallopian tube | Endometriosis, tubal damage, and infertility | 86 |
| Ovary | Premature ovarian failure | 87 |
| Placenta | Preeclampsia | 88 |
| Breast | Breast cancer | 45 |
| Eye | Keratoconus | 89 |
| Heart | Heart failure and arrhythmia | 90 |
| Connective tissue | Various degenerative changes | 91 |
The human skin is the body's largest organ, and an insight into the biology of the dermal TCs will improve our understanding of the basic mechanisms of cutaneous homeostasis.
The Distribution of TCs in the Normal Human skinHistologically, TCs are finely distributed throughout the dermis of normal skin. They appear as spindle-shaped cells. They are located mainly in the reticular dermis surrounding the blood vessels. The TCs form 2–3 incomplete concentric sheaths around the dermal structures, including the sebaceous glands, eccrine sweat glands, arrector pili muscles, and within the perifollicular sheath. In the papillary dermis, the density of the TCs is gradually reduced toward the dermo-epidermal junction. The epidermis is devoid of the TCs.11
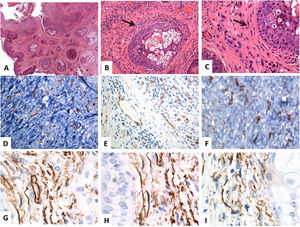
The Immunohistochemical Features of the Subtypes of the Cutaneous TCsThe immunophenotypic features of the cutaneous TCs differ according to their subtype and distribution. Two subtypes of cutaneous TCs have similar morphology but different immunophenotyping features. The first subtype is the CD34 and vimentin-positive TCs, which reside in the reticular dermis around sebaceous glands, hair follicles, arrector pili muscles, and sweat glands (Fig. 1). The second subtype is the CD117 and vimentin-positive TCs, which reside around the hair follicles and the sweat glands (Fig. 2). Some PDGFRα positive TCs reside in the papillary dermis.11
Histological and immunohistochemical (CD34) localization of the telocytes in normal skin. (A–C) Hematoxylin and eosin staining of the normal human skin, revealing unremarkable epidermis and dermal connective tissue. Within the dermal connective tissues, some spindle-shaped cells are finely distributed (arrows) throughout the dermis (original magnifications: A: ×40, B: ×200, C: ×400). (D–F) CD34 immunostain and the brown chromogendiaminobenzidine decorate the localization of several telocytes (TCs)/CD34-positive cells distributed throughout the dermis. The telocytes appear as spindle-shaped cells, having a nucleated oval or triangular cell body and thin, long, and varicose, moniliform tadpoles (original magnifications: D: ×400, E: ×400, F: ×600). (G–I) CD34-positive telocytes form an almost continuous layer around the basement membrane of the hair follicles (cells outer root sheath) and sebaceous glands (germinative layer) (original magnifications: G–I: ×1000, oil immersion).
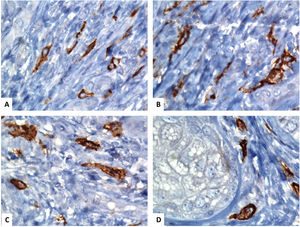
Immunohistochemical localization of the telocytes in the human normal skin using CD117 (C-KIT). (A–D) spindle-shaped telocytes (TCs) are stained by CD117and the brown chromogendiaminobenzidine. The TCs can be easily separated from the surrounding other stromal cells. The TCs have an elongated appearance with oval or triangular nuclei (counterstained with Mayer's hematoxylin) and long, varicose tadpole-like processes extending from their cell bodies (original magnification, ×1000, oil immersion).
The dermal TCs with their characteristic long extensions (Tps) have similar ultrastructural features to TCs described in the other organs. In the skin, the Tps exhibit alternating thin fibrillar-like segments (podomeres) and dilated, cistern-like segments (podoms), which contain the mitochondria, endoplasmic reticulum, and caveolae. The podomere/podom structure imparts a moniliform appearance on the Tps.11 Examining the 3D configuration of the dermal TCs using focus ion beam scanning electron microscopy (FIBSEM) revealed some interesting findings. They indicated that the TCs can shed microvesicles (>100nm) rather than exosomes (<100nm), and the Tps appear as uneven tubular-like structures with irregular dilations or variable ribbon-like segments.22
The Cytokine Expression Profile of the Cutaneous TCsThe TCs represent a distinct cell population that can be separated from other dermal stromal interstitial cells such as fibroblasts, dendritic cells, myofibroblasts, Langerhans cells using some immunohistochemical markers (Table 6). There are high expression levels of the epithelial neutrophil activated peptide (78ENA-78) and the granulocyte chemotactic protein (2GCP-2) cytokines in the TCs as compared to the stromal fibroblasts. The expression of these cytokines in the dermal TCs supports their roles in cutaneous homeostasis, angiogenesis, immune modulation, intercellular signaling, and carcinogenesis. Alternatively, the expression values of the cytokines involved in wound healing are higher in the dermal fibroblasts than in the TCs.46
The Immunohistochemical Variations Among the Cutaneous TCs and the Surrounding Interstitial Cells.
| Cell types | Immunohistochemical marker | References |
|---|---|---|
| Telocytes | PDGFRα and CD34 | 49 |
| Fibroblasts | Procollagen-1 | 46 |
| Endothelial cells | CD31 | 92 |
| Myofibroblasts | αSMA | 93 |
| Pericytes | PDGFRβ, αSMA, CD146, NG2, and nestin | 94 |
| Macrophages | CD68, CD11c, CD11b, CD64, CD40, and CD14 | 95 |
| Melanocytes | HMB45, S-100, and Melan-A | 96 |
| Langerhans cells | CD1a, S100, CD68, and Langerin | 49 |
| Stem cells | CD34, CD29, SCA-1, CD90, and CD44 | 97 |
| Dermal dendritic cells | CD83, CD11c, and CD208 | 49 |
| Plasmacytoid dendritic cells | CD205, CD11c, TNFα, and CD123 | 49 |
| Inflammatory dendritic cells | CD209, CD11c, NOS, and CD14 | 49 |
Although the exact biological functions of cutaneous TCs are still unclear, several studies have proposed some roles for these cells. The 3D configuration of the TCs maintains the microarchitecture of the dermis by supporting the networks between dermal collagen and elastic fibers.47 The TCs act as nursing cells for the epithelial and dermal mesenchymal stem cells; therefore, the dermal TCs are involved in skin repair and regeneration. This biological function is reasoned to the fact that the Tps of the TCs enwrap clusters of the dermal stem cells near the hair follicle bulge, and this direct interaction supports their role as nursing cells.48 The TCs play roles in immune modulation through the interaction of the dermal TCs through the heterocellular junctions with several dermal immune cells such as the macrophages and the mast cells. In support, the TCs are important immune modulators in some cutaneous allergic and autoimmune disorders.11 The TCs support skin repair and regeneration. These cells can maintain vascular integrity and angiogenesis through their direct interactions with dermal endothelial cells.49 Additionally, the TCs can modify the surrounding stromal cells. They control the fibroblasts's functions by secreting some paracrine signaling molecules such as microRNAs and shedding the extracellular vesicles.11
The Involvement of the Cutaneous TCs in Some DermatosesThe TCs seem to be involved in the development of some dermatoses, such as psoriasis vulgaris and systemic sclerosis.49,50 These disorders are associated with the dystrophy of the TCs and the reduction of their density in the lesional skin. In the psoriatic skin, there is dystrophy and a severe decrease in the number of TCs. Ultrastructurally, there are apoptotic nuclei, cytoplasmic disintegration, interrupted basement membranes, fragmented Tps, and loss of the homocellular junctions between TCs. The TCs in perilesional normal skin is structurally and numerically normal.49 In psoriasis vulgaris, there is a papillary dermal vascular ectasia (vascularized dermal papillae) due to the loss of the contractile function of the vascular smooth muscle cells. The loss of the TCs around the blood vessels is implicated in this vascular ectasia and the characteristic “Auspitz's sign” seen in psoriasis. The disruption of the basement membrane of TCs in psoriasis facilitates the migration of the Langerhans cells from the epidermis to the dermis resulting in the activation of T cells. The alterations of the interactions between the TCs network and the T-cell mediated immune reaction are essential in the initiation and progression of psoriasis.49,50
Systemic sclerosis is a multi-organ disease characterized by excessive fibrosis, inflammation, and vasculopathy (due to endothelial cell injury). It involves the skin, heart, lung, esophagus, and kidney.47 In systemic sclerosis, there is a loss of TCs and extensive fibrosis that disrupts the microanatomy of the dermis. The alterations of the TCs in systemic sclerosis include cytoplasmic vacuolation, swollen mitochondria, and lipofuscin bodies. These changes are reasoned to the ischemic damage of TCs resulting from the injury of the endothelial cells and oxygen deprivation. In the early phases of systemic sclerosis, the density of the TCs around hair follicles, sebaceous glands, arterioles, and nerves are reduced in the reticular dermis and are absent in the papillary dermis. The disease progression is associated with the loss of most dermal TCs except for a few cells around the eccrine sweat glands. The loss of TCs is reasoned to the associated ischemic changes.47 The TCs enwrap collagen and elastic fibers in the dermis, and therefore the alterations of the TCs also affect the extracellular matrix since TCs enwrap collagen and elastic fibers.47
The Involvement of the TCs in Cutaneous Squamous and Basal Cell CarcinomasTo date, our knowledge about the roles of the TCs in cutaneous carcinogenesis is largely unknown. It is well-known that a limitation of cell-cell contact is the hallmark of invasive carcinomas. Several observations support the roles of TCs in the development of cutaneous carcinomas. For instance, the presence of TCs in the tumor stroma of the cutaneous squamous cell carcinoma and basal cell carcinoma.51 In these carcinomas, there is a decreased density of the heterocellular junctions among the TCs and the peritumoral stromal cells. Alternatively, the homocellular junctions are preserved. The TCs-mast cell junctions are also lost, which results in the loss of control over the secretion of the mast's cell granules and the overexpression of inflammatory mediators in the tumor stroma.
Furthermore, TCs-endothelial cell junctions are lost in these tumors. The loss of the heterocellular junctions of TCs result from the loss of TCs functions in the tumor stroma. The TCs can exert paracrine function by shedding extracellular microvesicles in these tumors.51 These microvesicles transfer signals are required for cancer cell motility, tumor progression, and metastasis. Therefore, the TCs are involved in tumor development and progression.51,52
The Implications of TCs As a Targeted TherapyThe TCs play important roles in tissue repair, homeostasis, and regeneration through their communication with stem and progenitor cells. The TCs express stem cell markers (Sca-1, c-kit, and Oct 4), and as such, they represent promising future players in the field of regenerative medicine.9,53 The TCs also express VEGF and PDGFR-β, which promote angiogenesis during tissue repair processes.53 Therefore, the TCs represent a novel therapeutic target.54 Several observations support this notion. In a murine model of the partial hepatectomy, the TCs stimulate the proliferation and regeneration of hepatocytes and progenitor cells to restore liver size.55 The transplantation of TCs in an experimental model of asthma can improve airway hyper-responsiveness and inflammation through suppression of Th2 cell differentiation and stimulation of Th1 cells and their related cytokines. Therefore, transplantation of these TCs represents a new therapeutic strategy in bronchial asthma.56 In the experimental models of the myocardial infarction, the direct injection of the TCs into the damaged cardiac muscle was associated with regeneration and a decrease in the size of the infracted areas.57
To conclude, the TCs are novel interstitial cells widely distributed not only in the gut but also in most human organs. They have specific immunohistochemical and ultrastructural features that help separate them from other interstitial cells.
Several research issues should be addressed by future research such as (i) whether TCs represent a homogeneous population of cells or heterogeneous subpopulations of cells specific for each organ; (ii) what are the specific immunohistochemical markers of the TC to facilitate its identification; (iii) what is the exact role of TCs in the neoplastic and non-neoplastic conditions, and (iv) what are the therapeutic implications of TCs in human diseases. An improved understanding of these issues will help use the TCs as novel therapeutic targets in the future.
Conflict of InterestsThe authors declare that they have no conflict of interest.