Syringocystadenocarcinoma papilliferum (SCACP) is a very rare variant of cutaneous adnexal carcinoma that tends to present as an in situ malignant transformation of syringocystadenoma papilliferum (SCAP) before progressing to an invasive carcinoma. We present the case of an invasive SCACP with squamous differentiation.
The patient was a 40-year-old man with no relevant past history who presented with a progressively growing pigmented lesion on his chest that had appeared 1 year earlier. He reported that the lesion was asymptomatic, apart from occasional bleeding caused by friction.
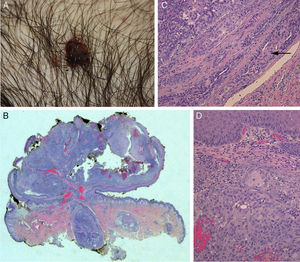
The physical examination revealed a pediculated erythematous-brownish papule with a 10-mm diameter covered by an exudative crust (Fig. 1).
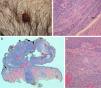
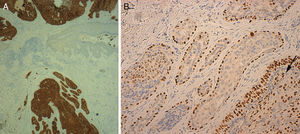
Histology showed a lesion with a mixed endophytic-exophytic growth pattern and cordlike growth in the exophytic portion. The lesion was in contact with the epidermis and expanded into dermis. It was formed by clear and squamous cells. The squamous cells had marked atypia and were limited by the basal membrane in some cases but invaded the dermis in others. The deepest portion, under the epidermal collarette, was formed by tubular structures surrounded by a fibrous band and containing 2 layers of cells. Focally, the structures were colonized by an atypical cellular component. The epithelial population on the surface was positive for cytokeratin 7 and 5/6 and negative for cytokeratin 20. p63 was positive in the superficial and deep portions of the tumor. In the areas of the tubular structures, p63 was positive only in the peripheral layer of myoepithelial cells. The atypical component was weakly positive for periodic acid-Schiff (PAS). These findings led to a diagnosis of invasive SCACP with squamous differentiation (Figs. 1 and 2).
A, Photograph of syringocystadenocarcinoma papilliferum. B, Panoramic view of the tumor showing a pediculated exophytic and endophytic lesion with a benign, well-delimited, round area at the base (hematoxylin-eosin, original magnification ×25). C, Detail of benign lesion formed by tubular structures partially colonized by atypical cells (arrow) (hematoxylin-eosin, original magnification ×100). D, Area of syringocystadenocarcinoma papilliferum with dermal invasion showing cells with squamous differentiation, mitosis, and pleomorphic nuclei (hematoxylin-eosin ×100).
A, Cytokeratin 7 staining with positive results for tumor cells and negative results in the epidermis (original magnification ×40). B, p63 positivity in peripheral myoepithelial cells in the benign area and in malignant cells infiltrating the tubular structures (arrow) (original magnification ×200).
Because the tumor was in contact with the lateral margin, the excision margins were widened by 1cm, but no traces of tumor were found.
After 8 months of follow-up, the patient showed no signs of recurrence.
SCACP is an adnexal carcinoma of controversial origin (apocrine vs eccrine) for which only 37 cases have been described. It is considered to be the malignant version of SCAP. It mainly occurs in the head and scalp area of patients older than 60 years and has no clear predilection for sex.1–5 It is a low-grade tumor. There have been just 6 reports of locoregional metastasis and no reports of metastasis to distant sites.4,5 Tumor recurrence has been observed in 3 patients.4 SCACP may occasionally grow multifocally with in situ and invasive areas within a SCAP or a sebaceous nevus2,4,6,7(Table 1).
Summary of Clinical and Histologic Characteristics of Syringocystadenocarcinoma Papilliferum Cases Published to Date.
| No. | Age, y | Sex | Location | Size, mm | Time Since Onset | In situ | Invasive | Locoregional Metastasis | Squamous Cell Differentiation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | F | Scalp | 65 | 30 y | + | |||
| 2 | 71 | F | Back | 30 | Months | + | |||
| 3 | 52 | F | Scalp | 65 | Congenital | + | + | ||
| 4 | 52 | F | Chest | 130 | 20 y | + | + | ||
| 5 | 47 | M | Scalp | 25 | – | + | |||
| 6 | 61 | M | Perianal region | 60 | 10 y | + | |||
| 7 | 64 | M | Scalp | 35 | 2 y | + | |||
| 8 | 60 | M | Outer ear | 40 | Since childhood | + | |||
| 9 | 81 | F | Scalp | 15 | – | + | |||
| 10 | 65 | M | Suprapubic area | 35 | 2 y | + | |||
| 11 | 83 | M | Perianal region | 15 | – | + | |||
| 12 | 77 | M | Leg | 25 | 9 y | + | |||
| 13 | 56 | F | Neck | 20 | 10 y | + | |||
| 14 | 58 | M | Forehead | 25 | – | + | |||
| 15 | 46 | F | Scalp | 35 | – | + | + | ||
| 16 | 67 | M | Scalp | 25 | – | + | |||
| 17 | 60 | F | Scalp | 30 | 30 y | + | + | ||
| 18 | 81 | M | Temple | 20 | – | + | |||
| 19 | 86 | F | Neck | 45 | 4 mo | + | + | + | |
| 20 | 62 | M | Axilla | 35 | 6 mo | + | |||
| 21 | 67 | M | Scalp | 40 | Since childhood | + | |||
| 22 | 83 | F | Upper arm | 30 | 7 y | + | + | ||
| 23 | 86 | M | Eyelid | 4 | – | + | |||
| 24 | 83 | M | Penis | 12 | – | + | |||
| 25 | 75 | F | Upper arm | 16 | 1 y | + | + | ||
| 26 | 66 | M | Scalp | – | 20 y | + | + | ||
| 27 | 66 | F | Scalp | 30 | 1 y | + | |||
| 28 | 65 | M | Scalp | 30 | Congenital | + | + | ||
| 29 | 32 | F | Scalp | 22 | – | + | |||
| 30 | 93 | M | Leg | 20 | 10 y | + | |||
| 31 | 74 | M | Scalp | 20 | Congenital | + | + | + | |
| 32 | 42 | M | Scalp | 45 | 3 wk | + | + | ||
| 33 | 80 | F | Scalp | 50 | Months | + | |||
| 34 | 88 | M | Shoulder | 15 | – | + | + | ||
| 35 | 76 | M | Perianal region | 16 | 6 mo | + | + | + | |
| 36 | 57 | F | Upper lip | – | 3 y | + | + | ||
| 37 | 60 | F | Scalp | 28 | 1 y | + | |||
| 38a | 40 | M | Chest | 10 | 1 y | + | + |
Parekh et al.4 proposed that some cases of SCACP might develop following the progressive accumulation of genomic alterations in a sebaceous nevus within the following sequence: sebaceous nevus, SCAP, SCACP in situ, and invasive SCACP. There is, however, no genomic evidence of this, and the theory is supported only by statistical associations.
Clinically, SCACP presents as an erythematous, skin-colored, yellow, or brown exophytic nodule that is typically asymptomatic (although it can be painful and/or ulcerated. It grows with variable speed and may even alternate between periods of stability and growth. Time to diagnosis ranges from several weeks to 30 years.4
Histologically, SCACP is characterized by glandular cells with decapitated cytoplasm and features of malignancy (pleomorphism and a high nuclear to cytoplasmic ratio) and positivity for PAS-diastase. There are 3 clinical presentations: 1) adenocarcinoma in situ, which has the characteristic architecture of SCAP in addition to a dysplastic epithelium separated from the adjacent dermis by a layer of basal or myoepithelial cells forming a “basal membrane”; 2) invasive adenocarcinoma, in which the dysplastic cells break through the basal membrane to invade the dermis and form neoplastic glands; and 3) foci of invasive squamous cell carcinoma in a SCAP.6
Immunohistochemistry can aid diagnosis. Cytokeratin 5/6 and p63 positivity (as in our case) helps to distinguish SCACP from metastatic adenocarcinoma and hidradenocarcinoma papilliferum, while cytokeratin 20 negativity helps to distinguish it from metastatic adenocarcinoma.2,3
SCACP is associated with multiple histologic features and variants, including basaloid areas, lymphoepithelioma-like features, squamous differentiation, pagetoid infiltration of the epidermis, perineural infiltration, psammoma bodies, sebaceoma, sebaceous carcinoma, and ductal in situ carcinoma.1–4 Considering this histologic complexity, it is advisable not to establish a histologic diagnosis unless it is possible to examine the whole structure.2
The differential diagnosis should include hidradenocarcinoma papilliferum, ductal apocrine adenocarcinoma, and skin metastases from adenocarcinomas at other sites.
There are no treatment protocols for SCACP. Surgery with wide margins is the current treatment of choice,8 although Mohs micrographic surgery has also been proposed.9 Sentinel lymph node biopsy with lymph node dissection has been performed in some cases.4,8 The use of chemotherapy and/or radiotherapy is controversial.5,10
In conclusion, we have presented a new case of SCACP, which is a very uncommon tumor. This is only the second case described in the chest area and the sixth with areas of squamous differentiation.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pérez ADA, Iriarte BB, Iturriagagoitia AC, Peñalba AV. Siringocistadenocarcinoma papilífero, descripción de un caso y revisión de la literatura. Actas Dermosifiliogr. 2018;109:366–369.