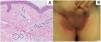
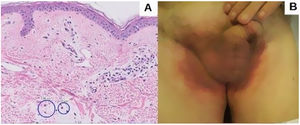
An 82-year-old man with a history of dyslipidemia, diabetes mellitus, and chronic ischemic heart disease developed a pinkish macular exanthema. The rash was pruriginous and without scaling or epidermal detachment and had appeared some weeks earlier. It initially affected the groin before extending posteriorly to the flanks and the back. The patient had changed his brand of esomeprazole 1 month earlier. Esomeprazole was withdrawn because of suspected toxicoderma, and topical treatment was prescribed with methylprednisolone aceponate cream 1mg/g. The patient's lesions resolved in 1 week. The laboratory workup revealed mild eosinophilia, and a skin biopsy showed a mainly lymphocytic superficial perivascular inflammatory infiltrate with mild edema, mild vascular ectasia, and occasional eosinophils (Fig. 1A). Prick testing was performed with both brands of esomeprazole, as was a challenge test. The results were negative. The lesions did not reappear when the patient reinitiated treatment with esomeprazole.
Five months later, the patient attended for recurrence of the lesions (Fig. 1B). Of note, he had received his third dose of the COVID-19 vaccine Comirnaty 48hours before onset of symptoms. On further questioning, he reported that the previous episode had occurred 72hours after receiving the second dose of Comirnaty.
Given the suspicion of symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) associated with administration of Comirnaty, prick testing was completed with the Comirnaty vaccine and with polyethylene glycol (PEG), and patch tests were performed with the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group, metals, special metals from the Martí Tor series, cosmetics, acrylates, and PEG. The results were negative in all cases. Challenge testing with the vaccine was not performed given the recent administration of the third dose. The patient received topical treatment with clobetasol cream 0.5mg/g, and the lesions resolved 4weeks later.
Based on the clinical and histopathological characteristics of the lesions, the temporal relationship with the administration of the vaccine, and the absence of other causes that could account for the clinical picture and considering the causality criteria of Naranjo1, the patient was diagnosed with “probable” SDRIFE induced by Comirnaty.
SDRIFE, which was previously known as baboon syndrome, is a type of systemic contact dermatitis. It manifests as symmetric erythematous exanthema that mainly affects the buttocks, genitals, and skinfolds on the extremities. It occurs when a person who is sensitized to a contact allergen is exposed systemically (transcutaneous, transmucosal, intravenous, intramuscular, oral, inhalation). It is a benign condition, with no manifestations of systemic toxicity and a good prognosis after withdrawal of the causative agent.2
Ten cases of SDRIFE caused by COVID-19 vaccine have been published: 5 with Comirnaty,3–6 2 with CoronaVac,6,7 and 3 with Vaxzevria/ChAdOx1 nCoV-19 (AstraZeneca-Oxford)8–10 (Table 1). The symptoms appear during the first few hours after exposure and up to several weeks later.2 In 7 cases, exanthema appeared after the second dose. No signs or symptoms of systemic involvement were observed in any cases.
Symmetrical Drug-Related Intertriginous Flexural Exanthema Reported After COVID-19 Vaccination.
| Vaccine | Age, y | Sex | Dose | Onset | Analysis | Biopsy | Skin tests |
|---|---|---|---|---|---|---|---|
| Comirnaty3 | 23 | M | Second | 6 wk | Eosinophilia | Vacuolar interface dermatitis. Mild spongiosisDIF negative | No |
| Comirnaty3 | 38 | F | Second | 2 wk | Normal | Eosinophilic spongiosisDIF negative | YesMild nickel+ |
| Comirnaty4 | 59 | M | Third | 2 d | Leukocytosis Neutrophilia | Superficial perivascular lymphocytic infiltrate with eosinophils | No |
| Comirnaty5 | 65 | M | ? | 2 wk | ? | ? | ? |
| Comirnaty6 | 52 | F | Second | 5 d | Normal | No | Negative patch/prick test results.Positive intradermal test results |
| CoronaVac6 | 57 | F | Second | 3 d | Normal | No | Negative patch/prick/intradermal test results |
| CoronaVac7 | 87 | M | ? | 4 d | Elevated renal profile parameters | Hyperkeratosis, acanthosis, spongiosis, lymphohistiocytic perivascular infiltration, extravasation of erythrocytes | No |
| Vaxzevria8 | 61 | M | Second | 1 d | Mild leukocytosis | No | No |
| Vaxzevria9 | 53 | M | Second | 10 d | Normal | No | No |
| Vaxzevria10 | 67 | F | Second | 5 d | Normal | No | Negative prick/intradermal test results |
Abbreviations: DIF, direct immunofluorescence; F, female; M, male.
Histopathologic findings for SDRIFE are nonspecific,3,4,7 and we see superficial perivascular lymphohistiocytic infiltrates that may include neutrophils or eosinophils.2 Spongiosis may or may not be present.
Cases of SDRIFE caused by vaccines whose components included thiomersal have been reported. Thiomersal was not a component of the COVID-19 vaccines reported. The adjuvant of Comirnaty studied as a possible cause was PEG.
Patch testing was performed in 4 of the cases of SDRIFE caused by COVID-19 vaccine described above,3,6,10 with a positive result for nickel in one.3 Prick testing yielded a negative result in 3 patients.6,10 Patch testing and prick testing can yield negative results, probably owing to poorer systemic absorption of the drugs when they are administered transcutaneously. Intradermal testing with the vaccine was positive in 1 case, although this positive response may have been the consequence of a local immune response in a patient who has already received the vaccine.6 Challenge testing yields positive results in most cases of SDRIFE.2 Challenge testing with COVID-19 vaccine has not been reported. All the patients responded well to topical or oral corticosteroids, with resolution of the skin lesions after a few weeks.
Cases of SDRIFE due to COVID-19 vaccine have been reported since the worldwide roll-out of the vaccination program. We report the case of a patient who developed SDRIFE after his second and third doses of the Comirnaty vaccine.
Conflicts of InterestThe authors declare that they have no conflicts of interest.