Actinic keratosis is a precursor lesion to the most common nonmelanoma skin cancer. Conventional photodynamic therapy (PDT) has been shown to be effective, but the procedure is time-consuming, can be very painful, and requires infrastructure. These shortcomings led to the emergence of daylight PDT. To obtain a global estimate of efficacy, we undertook a systematic literature review and performed a meta-analysis of the available evidence on the efficacy and safety of daylight PDT as compared to conventional PDT in the treatment of actinic keratosis and/or field cancerization. The conclusion is that the difference in efficacy is clinically negligible (global estimate of the mean response rate difference,–3.69%; 95% CI,–6.54% to–0.84%). The adverse effects of daylight PDT are mild and localized (79% of patients report no discomfort), and patients report less pain (P<.001). Daylight PDT gives good to excellent cosmetic results in more than 90% of patients, and patient satisfaction is greater (P<.001).
La queratosis actínica es la lesión precursora de cáncer cutáneo no-melanoma más frecuente. La terapia fotodinámica convencional se ha empleado eficazmente pero requiere tiempo, infraestructuras y es en ocasiones muy dolorosa. En este contexto surge la terapia fotodinámica con luz de día (TFDLD). Con el objetivo de estudiar las evidencias disponibles que evalúan la eficacia y seguridad de la TFDLD frente a la terapia fotodinámica convencional en el tratamiento de pacientes con queratosis actínica/campo de cancerización, y obtener un estimador global de eficacia, realizamos una revisión sistemática de la literatura y un metaanálisis. Se concluye que la variación en eficacia entre ambas terapias es clínicamente irrelevante (estimador global de la diferencia de tasas de respuesta media:–3,69%, IC 95%:–6,54 a–0,84). Con TFDLD el dolor referido es menor (p<0,001), los efectos adversos locales y leves (el 79% no refiere molestias), los resultados cosméticos buenos-excelentes (>90% de los casos) y la satisfacción del paciente mayor (p<0,001).
Actinic keratosis (AK) is the most common precancerous skin tumor. It consists of an intraepithelial keratinocytic dysplasia caused by cumulative exposure to UV light and can lead to in situ or invasive squamous cell carcinoma.1,2 The lifetime risk of AK is 50%,3 and this condition is among the most frequent reasons patients visit a dermatologist, absorbing both time and resources.
The known risk for progression to squamous cell carcinoma and the scarcity of evidence regarding prognosis that could indicate which lesions will progress make it necessary to treat all AKs.4,5 Molecular studies of tissue from the region where AKs emerge have found oncogenic mutations secondary to UV radiation that develop over time to display cell atypia and eventually form AKs or squamous cell carcinoma.6 Such studies have informed our understanding of the concept of field cancerization and suggest that the best treatment approach is one that targets the entire field.
Conventional photodynamic therapy (PDT), used increasingly for over 10 years, has become the treatment of choice for AK and field cancerization7 given response rates of 86% clearance at 4 months, recurrence rates under 20%,8 and excellent cosmetic results. Incubation with a photosensitizing agent increases the production of selective protoporphyrin IX (PpIX) by tumor cells. Exposure to light at the appropriate wavelength and dose in the presence of oxygen induces oxidation and cell death.9 This treatment requires dedicated staff, facilities, irradiation equipment, and time. However, the main problem with this modality is pain, and even though steps are taken to alleviate it, treatment must occasionally be interrupted.
Daylight-mediated PDT, which uses direct and air-reflected sunlight, has emerged in response to the need for a simpler and less painful therapy. A role for using natural light to induce a reaction in photosensitized skin was suggested in 2006,10 but it was in a 2007 publication that Batchelor et al.11 first documented the use of this modality and its effectiveness in considerably reducing the total number of AKs on a patient's scalp.
Later, a randomized controlled trial published by a group led by Wiegell in 2008 compared response rates and adverse effects of PDT with red light versus daylight in 29 patients with AK. They found no significant differences in efficacy between the 2 treatments (P=.13). Erythema and crust formation were also similar, but pain was significantly greater when red light was used (P<.0001). This group later suggested that slower activation of the small amounts of PpIX produced gradually in daylight PDT might be less painful than rapid photooxidation of the large amounts of PpIX produced during conventional incubation.12 A 2009 publication showed that a lower concentration (8%) of the photosensitizing agent (methyl aminolevulinate [MAL]) than the one used in the earlier studies (16%) could also be effective, and they established 8J/cm2 as the minimum effective light dose.13 The group then undertook a multicenter trial that was published in 2011.14 Two durations of daylight exposure (1.5hours and 2.5hours) were found to have statistically similar outcomes (response rates, P=.7; pain intensity, P=.94) and adverse effects. Seventy-two percent of patients in the trial were satisfied with daylight PDT. A trial by another group concluded that although the response to daylight PDT was significantly greater when AK was mild (P<.0001), 86% of type II AKs and 94% of type III AKs nonetheless cleared completely or were reduced to a lower grade.15 Other authors reported similar results in Switzerland,16 Brazil,17 and southern Italy.18
After several years of uncertainty and lack of consensus about daylight PDT, given the scarcity of evidence, new phase III clinical trials were recently published.19,20 The aim of the present systematic review was to study the available evidence for the efficacy and safety of daylight PDT in comparison with conventional PDT for treating AK and field cancerization. We evaluated the consistency and quality of the trials published and meta-analyzed the results to obtain a global estimate of effect.
MethodsThis systematic review and meta-analysis took into consideration the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA recommendations).21
Sources and Search StrategyOur sources of articles were MEDLINE, the Cochrane Central Register of Controlled Trials, the Web of Science, and the registry of clinical trials of the US National Institutes of Health.
The first search was done in June 2015. The same search strategy was repeated in December 2015. We used the following search terms: actinic keratoses and daylight photodynamic therapy. In the PubMed portal we added the term randomized or randomised. No year or language restrictions were introduced, and we read articles in both English and Spanish. Full texts were accessed. In addition, we consulted experts and contacted one of the authors of the included studies to be sure we had not missed any important trials and to check that we had understood certain trial characteristics and analyzed them correctly. We also consulted the gray literature to avoid publication bias. The reference lists of all studies found were also reviewed.
Inclusion CriteriaInclusion was restricted to randomized controlled trials that evaluated the efficacy or noninferiority of daylight PDT versus conventional PDT in humans with AK. This decision was based on the need to determine that daylight PDT is no less effective than conventional PDT and that it was better tolerated by the patient, being simpler and more cost-effective.
Any population, age group, or body area exposed could be included. Duration of follow-up was not a selection criterion, and no restrictions were placed on the PDT protocol followed.
Article Selection Process, Data Items and Extraction, and Analysis of Quality of Trial DesignWe first completed the search, evaluated the studies, and synthesized their results.
The 2 main variables established a priori were lesion response rate and pain caused by the procedure. Other variables, such as AK grade, other adverse effects, patient satisfaction, cosmetic results, duration of follow-up, statistical approach, or blinding were also taken into account.
The search was unblinded with regard to authors, journals, and research facility.
We consulted the Jadad scoring system for randomized trials,22 and our critical analysis was based on the CONSORT reporting guidelines.23
Statistical AnalysisThe meta-analyzed variable was the percentage of lesions with a complete response (number of fully cleared lesions as a percentage of the number treated) at week 12. We calculated the difference between the mean rate of response to daylight PDT and the mean rate of response to conventional PDT; we also calculated 95% CIs. A fixed-effects meta-analysis was performed according to the inverse variance method. Both per-protocol (PP) and intention-to-treat (ITT) analyses were carried out. Noninferiority of efficacy of daylight versus conventional PDT was demonstrated based on the expert-established thresholds for a clinically significant difference in effect between treatments: 20% according to Rubel et al.19 and 15% according to Lacour et al.20
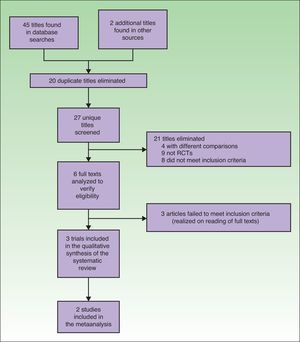
ResultsTrial SelectionTwenty articles were identified in the MEDLINE search, 14 in the Cochrane database, and 11 in the Web of Science. Two more gray literature articles were found, for a total of 47 articles in all. Twenty repeated titles were discarded. Of the 27 remaining, 21 were discarded because they clearly failed to meet the inclusion criteria. Four compared aspects of PDT that were not of interest24–27 (photosensitizing agents vs placebo, effectiveness of different photosensitizing agents, artificial daylight vs red light-emitting diodes [LED] vs daylight vs white LED), 9 were not randomized trials (3 expert consensus reports or international association opinions,28–30 3 qualitative reviews,17,31,32 1 retrospective case series,16 1 letter to the editor,33 and 1 conference summary34). The remaining 8 articles also discussed issues different from the objective of our study (the use of different photosensitizing agents,35 photosensitizer concentration required for effective daylight PDT,36 cost-effectiveness of daylight PDT versus LED PDT,37 fractional ablative laser treatment in combination with daylight PDT,38 effect of applying a sun screen to reduce inflammation after daylight PDT,39 effect of weather on daylight PDT in Australia,40 PDT results in transplanted patients,41 and PDT for head and neck tumors42). Following in-depth study of the 6 remaining articles, 3 more were excluded because they did not have the aims required for inclusion.13–15
Finally, 3 randomized trials comparing daylight PDT to conventional PDT were included in this systematic review.12,19,20 One of them12 was excluded from the meta-analysis (Fig. 1).
Trial and Participant CharacteristicsThe characteristics of all 3 trials qualitatively reviewed are shown in Table 1.
General Characteristics of the Trials Included in the Qualitative Analysis.
| Wiegell et al.12 | Rubel et al.19 | Lacour et al.20 | |
|---|---|---|---|
| Objective | To compare conventional PDT to red light PDT to daylight PDT (response rates and adverse effects) | To evaluate the noninferiority of daylight PDT vs conventional PDT and intensity of pain caused | To demonstrate the noninferiority of efficacy and the superiority of safety of daylight PDT vs conventional PDT |
| Journal and year of publication | Br J Dermatol 2008 | Br J Dermatol 2014 | J Eur Acad Dermatol Venereol 2015 |
| Trial design | Randomized, controlled, single blind | Phase III trial, multicenter, randomized, controlled, single blind | Phase III trial, multicenter, randomized, controlled, single blind |
| Country | Denmark | Australia | France, Germany, Spain, Switzerland, the Netherlands |
| No. of centers | 1 | 7 | 18 |
| Analytic approach | NS | PP, ITT of efficacy | PP, ITT of efficacy |
| Follow-up, wk | 12 | 24 | 12 |
| Characteristics of the patient sample: | |||
|---|---|---|---|
| Wiegell et al.12 | Rubel et al.19 | Lacour et al.20 | |
| No. of cases | 29 | 100 | 108 |
| Age, range (mean) | 63–90 (78) | 42–90 (66) | 47–91 (72.8) |
| Sex, M/F | 23/6 | 75/25 | 99/9 |
| AK grade | I,II,III | I,II | I,II |
| Location | Face, scalp | Face, scalp | Face, Scalp |
| Surface area treated | 80cm2 | Minimum 5 AKs, maximum 8×18cm | Minimum 5 AKs, maximum 6×16cm |
| Characteristics of the PDT procedure used in the trials: | |||
|---|---|---|---|
| Wiegell et al.12 | Rubel et al.19 | Lacour et al.20 | |
| When administered | June–September, 2006 | March–November, 2012 | July–January, 2013–2014 |
| No. of sessions | 1 | 1 | 1 |
| Sunscreen during daylight PDT | None | High SPF, without mineral screens | High SPF, with chemical screens |
| Photosensitizing agent | MAL 16% | MAL 16% | MAL 16% |
| Incubation | 30min in daylight PDT, 3h in conventional PDT | 30min in daylight PDT, 3h in conventional PDT | 30min in daylight PDT, 3h in conventional PDT |
| Duration of exposure in daylight PDT, h | 2.5h | 2h | 2h |
| Weather conditions during daylight exposure | Sunny, partly cloudy | Sunny, partly cloudy | Comfortable outdoor conditions, not raining |
Abbreviations: AK, actinic keratosis; F, female; ITT, intention-to-treat analysis; M, male; MAL, methyl aminolevulinate; NS, not specified; PDT, photodynamic therapy; PP, per-protocol analysis; SPF, sun protection factor.
These 3 all treated 2 symmetrical areas in the same patients (intraindividual comparison) and used MAL as the photosensitizing agent. After application of MAL, daylight exposure of 1 of the areas started before 30minutes had elapsed; exposure continued for 2to 2.5hours. On the comparator area, the photosensitizer was left incubating for 3hours, after which the skin was exposed to red light.
Trial QualityThe results of our analysis of quality based on the CONSORT reporting guidelines for randomized clinical trials23 were as follows.
The method used to measure adverse effects could have been made more explicit in the study by Wiegell et al.12 These authors could also have explained their inclusion criteria more specifically and added a table showing patients’ baseline characteristics. In addition, they did not specify how the randomization sequence was generated, how researchers were blinded, or whether the dermatologist who assessed the response was blinded. CIs were not calculated and limitations were not listed.
The other 2 trials, published in 2014 and 2015, shared the same design features. They were randomized investigator-blinded controlled trials lasting 24 weeks (Rubel et al.19) and 12 weeks (Lacour et al.20). They both compared intraindividual efficacy (noninferiority) and safety (superiority in terms of less pain) of daylight PDT versus conventional PDT for facial/scalp AK. Their aims, the design, and criteria were clear and specific. Tables describing the sample and interventions were provided, and the authors explained how variables were assessed. Statistical power was reported and the randomization and blinding procedures were explained. They included both PP and ITT analyses, calculated 95% CIs, described adverse effects, and listed limitations (simple blinding,19,20 the difficulty of generalizing findings because of climate differences,19 and the follow-up time20). Both complied with ethical standards.
Only the researchers were blinded in all 3 trials. Pain is a subjective, patient-reported variable and a patient's answers might change if the aim of the study and treatment assignment is acknowledged, possibly biasing the information that can be obtained.
The information in each of the studies is summarized in Table 2.
Characteristics Found in the 3 Trials Selected.a
All 3 trials were registered at clinicaltrials.gov.
Trial ResultsTable 3 shows the findings of each of the 3 trials reviewed.
Results of the Qualitative Analysis of the 3 Selected Trials.
| Wiegell et al.12 | Rubel et al.19 | Lacour et al.20 | |
|---|---|---|---|
| Results at 3 mo | Reduction of 79% of AKs with daylight PDT and 71% with conventional PDT (P=.13) | Complete response rates: PP, 89.2% with daylight PDT; 92.8% with conventional PDT (95% CI, −6.8% to −0.3%) ITT, 86.4% with daylight PDT; 89.9% with conventional PDT (95% CI, −6.6% to −0.4%) | Complete response rates: PP, 70% with daylight PDT; 74% with conventional PDT (95% CI, −9.5% to 2.4%) ITT, not specified (95% CI, −8.6% to 2.4%) |
| Results at 6 mo | Not studied | Maintenance of complete response: 96% with daylight PDT; 96.6% with conventional PDT | Not studied |
| Pain scorea, mean (SD) | 2(1.9) with daylight PDT; 6.7 (2.2) with conventional PDT (P<.0001) | 0.8(1.2) with daylight PDT; 5.7 (2.3) with conventional PDT (P<.001) | 0.7 with daylight PDT; 4.4 with conventional PDT (P<.001) |
| Other findings | Adverse effects common to both treatments: erythema, crusts Patient preference: 62% preferred daylight PDT | Adverse effects by modality (% daylight PDT; % conventional PDT) Total (39%; 59%) Severe (0%; 0%) Interruption (0%; 0%) Skin reaction (24%; 30%) Erythema (2%; 5%) Phototoxicity (5%; 10%) Crust formation (9%; 9%) Bleeding (2%; 3%) Irritation (1%; 2%) Facial edema (1%; 0%) Itching (0%; 1%) Burning (0%; 2%) Edema (0%; 1%) Researcher's preference regarding tolerance: 48%, no preference 33.7%, daylight PDT; 18.3%, conventional PDT (P<.011) Clinical evaluation, at 12 wk 90% of treated lesions good or excellent Cosmetic results satisfactory and similar | Adverse effects: 45.4% with daylight PDT; 61.1% with conventional PDT (erythema, crusts, burning sensation) Cosmetic results at 12 wk: good or excellent, and similar (98% with daylight PDT; 99.7% with conventional PDT) Patient preferences (daylight PDT; conventional PDT): Satisfaction (64.8%; 18.9%) Pain-free (91.3%; 22.3%) No discomfort (79.4%; 43.4%) |
Wiegell et al.12 found that the 2 modalities had statistically similar levels of efficacy (P=.13) and saw that response to daylight PDT did not vary with light intensity.
Rubel et al.,19 who established 20% as the margin of difference to demonstrate noninferiority, found that the rate of complete response to daylight PDT (89.2%) was not statistically inferior to the response to conventional PDT (92.8%) at 12 weeks (difference,–3.6%; 95% CI, −6.8% to −0.3%). Moreover, clearance was maintained by 96% of the mild lesions at 24 weeks. The findings of Lacour et al.20 were consistent with the other 2 trials. Using a noninferiority margin of 15%, they observed complete response rates of 70% for daylight PDT and 74% for conventional PDT (difference,–4%; 95% CI, −9.5% to 2.4%). They also concluded that daylight PDT was effective in different weather conditions (Table 4).
Differences in Response Rates Between Conventional and Daylight PDT.
| Rubel et al.19 | Lacour et al.20 | Global Estimate | |
|---|---|---|---|
| Difference in per-protocol analysis | −3.6% 95% CI, −6.8% to −0.3% | −4% 95% CI, −9.5% to 2.4% | −3.69% 95% CI, −6.54% to −0.84% |
| Difference in intention-to-treat analysis | −3.5% 95% CI, −6.6% to −0.4% | −3.10% 95% CI, −8.6% to 2.4% | −3.40% 95% CI, −6.10% to −0.70% |
Abbreviation: PDT, photodynamic therapy.
Thus, daylight PDT is effective and not inferior to conventional PDT.
Patient-Reported PainPain was significantly greater in the area treated with conventional PDT (P<.0001) in the trial of Wiegell et al.12; although that result was unrelated to the level of PpIX fluorescence (P=.065), it was related to the effective light dose received (P=.041). Patients in the Australian19 and European20 trials found daylight PDT to be nearly painless and certainly less painful than conventional PDT (P<.001).
Adverse EffectsWiegell et al.12 reported that erythema and crusts formed after both the daylight and conventional PDT treatments to a statistically similar degree and that the effects did not differ between treatment areas in 38% of the patients.
Adverse effects in the phase III trials19,20 were all dermatologic and mild. The most common effect was a skin rash, and in one of the trials 79% of patients treated with daylight PDT were not inconvenienced by any adverse effect.20
Thus, daylight PDT does not have adverse effects that cause discomfort to patients.
Patient SatisfactionSixty-two percent of the patients treated by Wiegell et al.12 preferred daylight PDT. Satisfaction with this modality was also greater (P<.001) in the Australian trial.19 In the European trial, 64.8% of the patients were highly satisfied with daylight PDT (vs 18.9% with conventional PDT).
Cosmetic ResultsCosmetic results after both treatment modalities were rated good or excellent by 90% to 99.7% of the patients in the 2 phase III trials.19,20
Meta-analysisWe meta-analyzed the results of only the 2 phase III trials.19,20 The earliest trial reviewed12 was excluded from the meta-analysis because it used a phase II design whose objective was mainly photobiologic, to open doors to the use of this modality by demonstrating the continuous and effective production of PpIX in daylight.
The 2 later trials included a total of 186 patients in the PP analysis and 208 in the ITT analysis.19,20 A total of 4668 AKs were treated, 2336 with daylight PDT and 2332 with conventional PDT.
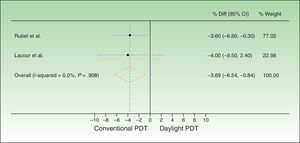
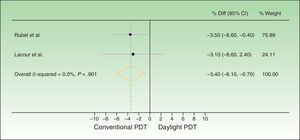
The difference between response rates (PP analysis) was 3.6% in the Australian trial19 and 4% in the European20 one. The global estimate of the mean response rate difference was −3.69% in favor of conventional PDT. Because the estimate of effect size in the Australian trial was closer to the estimated effect derived from meta-analysis, greater weight was assigned to this trial (77.02% vs 22.98% to the European trial). The I2 test result was 0.0%, as heterogeneity was very low between the 2 trials, whose design and results were similar (Fig. 2). Therefore, we did not perform further measures of heterogeneity. The results of the ITT analysis were similar, yielding a global effect estimate of 3.4% in favor of conventional PDT (Fig. 3).
Forest plot comparing response rates for conventional versus daylight PDT according to per-protocol analysis. The figure shows that the CIs were below the noninferiority margins established a priori (20% by Rubel et al.19 and 15% by Lacour et al.20). Thus, daylight PDT can be considered noninferior. PDT refers to photodynamic therapy and diff. to difference.
Forest plot comparing response rates for conventional versus daylight PDT according to intention-to-treat analysis. The figure shows that the CIs were below the noninferiority margins established a priori (20% by Rubel et al.1919 and 15% by Lacour et al.20). Thus, daylight PDT can be considered noninferior. PDT refers to photodynamic therapy and diff. to difference.
Thus, although conventional PDT is associated with higher response rates than daylight PDT, the difference is not clinically important, as it is less than the difference margins (20% and 15%) established a priori. The 95% CIs of the global effect estimates ranged from −6.54% to −0.84% and −6.10% to −0.70% in the PP and ITT analyses, respectively. Therefore, daylight PDT can be considered noninferior to conventional PDT.
DiscussionOur review of the available randomized trials comparing daylight to conventional PDT in patients with AK or field cancerization and our meta-analysis of 2 of the trials support the conclusion that the new daylight PDT modality is not inferior to conventional one. The strictest of the expert-proposed definitions of a clinically important difference is 15%. The global effect estimate of–3.9%, favoring conventional PDT, was within this margin and its 95% CI was −6.54% to −0.84%.
One factor that might contribute to the lower daylight PDT response rates of 89.2%19 and 70%20 (vs 92.8%19 and 74%20 for conventional PDT) is the lack of clinical control over the amount of daylight exposure.32 This theory is supported by a larger difference in effect, in favor of the conventional modality, in the PP analysis in comparison with the ITT analysis since patients interrupted conventional PDT because of pain.
The differences in efficacy and adverse effects between the trial of Wiegell et al.12 and the Australian19 and European20 trials may be attributable to the phase design, the first being a phase II trial and the other 2 being phase III trials. The first trial sought preliminary information to support efficacy, the presence of a dose-response pattern, and safety, whereas the 2 more recent trials compared the efficacy of the new modality to one that was already available.
Furthermore, some of the limitations authors initially hypothesized for daylight PDT have been attenuated since it has proven effective in almost any type of weather except rain, for the sake of patient comfort, and cold (below 10°C), to guarantee proper generation of PpIX.29 Likewise, adverse effects, which are all superficial and mild, do not differ greatly between daylight and conventional PDT. Subjective reports of pain on a scale of 0 to 10, however, were significantly lower with daylight PDT in all 3 of the trials we reviewed.12,19,20 Thus, patients tolerate daylight PDT better, prefer this modality, and express greater satisfaction with it.
AK is a prevalent and recurring problem that may progress to squamous cell carcinoma. Therefore, treatments that act quickly and whose effect is maintained are sought.19 Given that daylight PDT is noninferior with regard to efficacy but is better tolerated than conventional PDT, the new modality has been said to offer “gain without pain.”16 One recent study found that 70% of clinicians agreed that the duration of therapy and local reactions diminished adherence to treatment.43 Daylight PDT addresses the problem of risk of malignancy in AK by offering a more cost-effective alternative37 with savings in time, staff, material, and infrastructure. Nevertheless, vigilance after approval of daylight PDT to treat AK is important. The results obtained in controlled clinical trials in which patients have received extensive explanations and even been supervised during daylight exposure may differ from those achieved in routine clinical practice, where patients are often of advanced age.
It is true that response rates are lower in higher grade AK (grade I, 75.9%; grade II, 61.2%; and grade III, 49.1%).15 However, it is also true that all thick AKs become thinner after a single daylight PDT session.15 According to expert opinion, grade III AKs that might be present in field cancerization can be treated, even though it is assumed that their response will be less marked and that more sessions or adjuvant treatments may be needed.29
Thus, daylight PDT can be considered a first-line therapy for immunocompetent patients with grade I or II AK or field cancerization on the face or scalp according to the consensus statement of European Society for Photodynamic Therapy in Dermatology.29
Use of this modality in transplanted patients or other immunocompromised individuals can be taken under advisement, but further study will be needed in this population, which is particularly susceptible to the development of AK and nonmelanoma skin cancer. We also lack evidence of the efficacy of daylight PDT on other skin lesions, although anecdotal reports of its application in basal cell carcinoma,44 Bowen disease,45 and actinic cheilitis46 have been published.
The trials we selected have certain design flaws, such as the lack of specification of the randomization and blinding methods in the Danish study,12 and the short duration of follow-up (3 months) in both that study and the European multicenter one.20 However, the Australian study found that outcomes were satisfactory at 6 months.19 Bias that might result from lack of patient blinding when pain is reported has already been mentioned, but complete patient blinding in PDT is practically impossible. Therefore, the trials included in the meta-analysis can be considered to have been very well designed, lending support to their internal validity and, in consequence, to the global estimate of efficacy. The fact that only 2 trials could be included in the meta-analysis, however, makes it difficult to draw conclusions and reveals the need to continue to study daylight PDT.
A difficulty we encountered was the lack of sufficient information on the intensity of reported pain and lack of access to data. Such information would have been useful for calculating a global effect estimate of this adverse effect. The differences found, however, show a high level of clinical significance in the analyzed trials,19,20 where pain ranged from “sometimes unbearable” to “practically painless” and the Wilcoxon test found daylight PDT to be significantly superior (P<.001) in this respect.
To avoid publication bias, we searched for both published and unpublished trials on known databases, reviewed reference lists, and contacted experts in the management of daylight PDT. The trials we found, therefore, seem to represent the scope of research on this topic.
The external validity and applicability of the results are supported to a certain extent by the fact that the 2 meta-analyzed trials were carried out in geographic areas with different climates.
ConclusionDaylight PDT is not inferior to conventional PDT in the treatment of mild to moderate AK and field cancerization. The efficacy of this modality does not depend on weather conditions and has been applied at different latitudes. The procedure is practically painless, is better tolerated than conventional PDT, and does not have important adverse effects. Cosmetic outcomes are very good and patient satisfaction is high. Daylight PDT's profile of efficacy, tolerability and safety will probably make this modality a treatment of choice for AK and field cancerization.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We are grateful to Dr Alfredo Gea for his help with aspects of the statistical analysis and performance of the meta-analysis.
Please cite this article as: Tomás-Velázquez A, Redondo P. De la terapia fotodinámica convencional a la terapia fotodinámica con luz de día en el tratamiento de las queratosis actínicas: revisión sistemática y metaanálisis. Actas Dermosifiliogr. 2017;108:282–292.