Hidradenitis suppurativa is a chronic inflammatory skin condition characterized by painful nodules, abscesses, and sinus tracts that may lead to irreversible scarring complications. Therapeutic options include antibiotics, biologic therapies, and surgical procedures. Current management of hidradenitis suppurativa favors early surgical intervention along with medical therapy to promote healing and minimize scars and complications in a disease characterized by a therapeutic window of opportunity. Surgical techniques range from incision and drainage to wide excision, with varying recurrence rates mainly based on the extent of procedures. Reconstruction techniques would vary primarily based on the extent of the defect and the area involved. In all cases, a good preoperative planning and delimitation with imaging modalities, preferably intra- or perioperative facilitates complete removal of involved tissue, preserving the integrity and function of healthy skin and minimizing recurrences.
La hidradenitis supurativa es una enfermedad inflamatoria crónica de la piel caracterizada por nódulos dolorosos, abscesos y tractos fistulosos que pueden dar lugar a complicaciones cicatriciales irreversibles. Dentro de las opciones terapéuticas se incluyen el tratamiento con antibióticos, las terapias biológicas y los procedimientos quirúrgicos. El tratamiento actual de la hidradenitis supurativa promueve una intervención quirúrgica temprana junto con el uso de la terapia médica, con lo que se busca favorecer la curación y minimizar las cicatrices y complicaciones en una enfermedad que se caracteriza por una ventana de oportunidad terapéutica. Las técnicas quirúrgicas van desde la incisión y el drenaje hasta la escisión amplia, con tasas de recurrencia variables basadas principalmente en la extensión de los procedimientos. Las técnicas de reconstrucción varían principalmente en función de la extensión del defecto y de la zona afectada. En todos los casos, una buena planificación y delimitación preoperatoria con pruebas de imagen, preferiblemente intra o perioperatoria facilita la extirpación completa del tejido implicado, preservando la integridad y función de la piel sana y minimizando las recidivas.
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin condition characterized by nodules, abscesses and sinus tracts that result in disfiguring irreversible scarring in skinfold regions.1 HS causes severe pain and discomfort, leading to a significant impairment of the patients’ quality of life, social interactions and work functioning.2 Although the exact pathogenesis of HS is not fully understood, it is generally considered an autoinflammatory keratinization disease arising in the hair follicle epithelia of the infundibular region, which leads to the occlusion of hair follicles and secondary inflammation.3
Multiple treatment options exist for HS, including antibiotics, anti-inflammatory agents, immunomodulatory biologic drugs, para-surgical procedures or surgery, along with adjuvant measures such as pain management, tobacco cessation, or weight loss.4 The clinical heterogeneity of HS,5 its varying forms and rates of progression,6 and the wide range of medical or surgical therapeutic possibilities7 turn the choice of optimal therapy into a clinical challenge. This narrative review aims to summarize the surgical treatment options for the management of HS.
When is the optimal time for surgical intervention?Patients with HS face 2 main concerns: inflammation-related issues such as inflamed nodules and abscesses, typically managed with medical or non-surgical procedures; and secondary complications such as fistulous tracts and irreversible scarring, often requiring surgical intervention.7 Surgical treatment has traditionally been spared for recurrent localized lesions, medical therapy-resistant inflammatory lesions, or disease-related structural sequelae.7
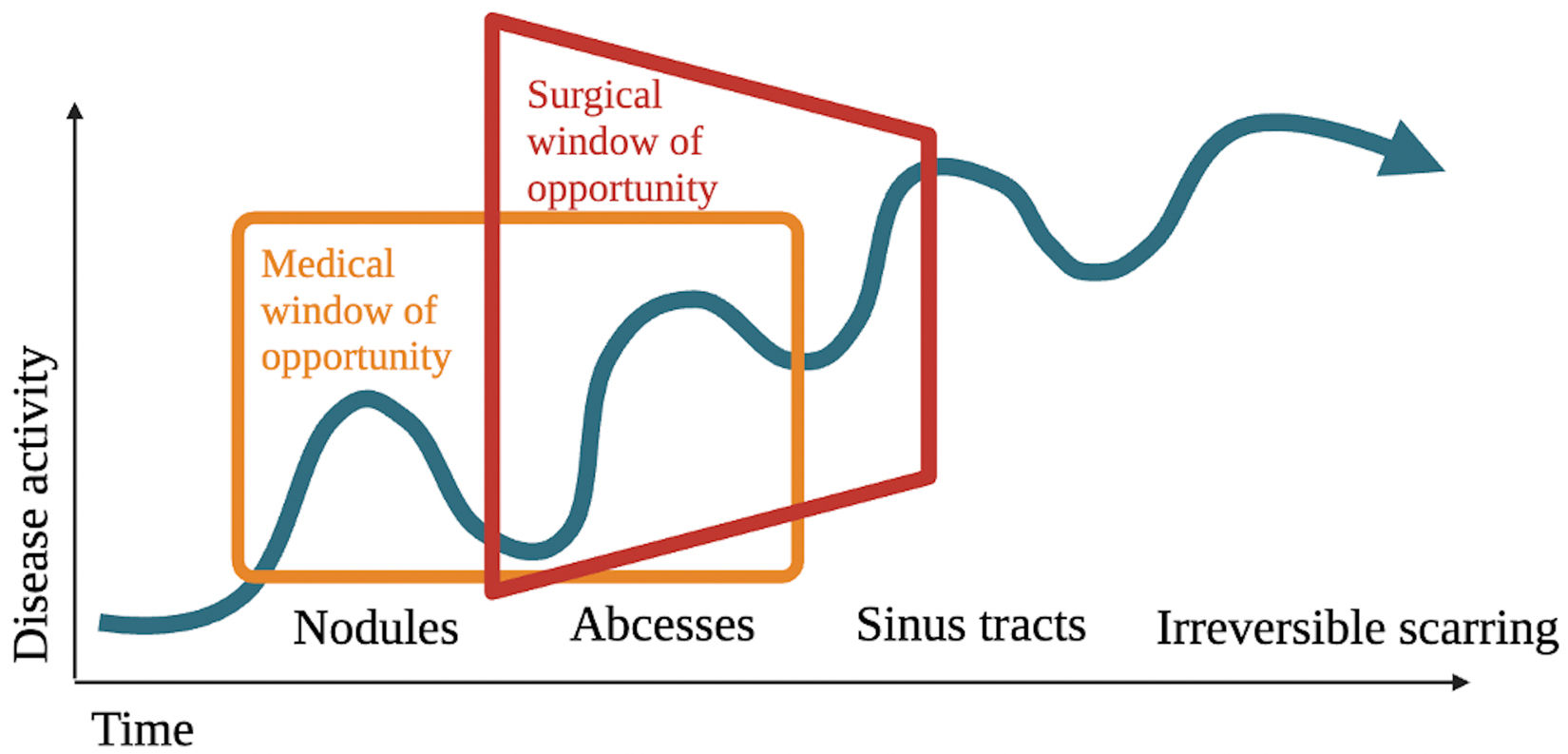
In recent years, earlier medical therapy has been suggested to improve responses and reduce scarring complications, leading to the concept of the window of opportunity in the treatment of HS.7 Manzano et al. conducted a real-world retrospective multicenter study including data from 389 patients and found that a longer time since HS onset to adalimumab initiation was a significant risk factor for being non-responsive to adalimumab in the univariate analysis (p=0.0016).8 Better outcomes have been reported with adalimumab+surgery vs surgery alone.9,10 Adalimumab reduces inflamed areas, decreasing the area requiring surgical removal. Medical therapy prior to surgery facilitates clearer differentiation between involved and healthy tissue during surgery while priming the wound for optimal healing.11 However, systemic therapy alone may not induce complete remission of inflammation in sinus tracts,10 and complete remission is essential to prevent residual skin inflammation inducing new inflamed lesions.12 Therefore, combining medical therapy + surgery could represent the best therapeutic standard. The window of opportunity concept would apply not only to medical therapy but also to surgery (Fig. 1). Early surgery of symptomatic inflamed early fibrotic lesions—along with medical therapy—could reduce surgical area, prevent relapse, and halt disease progression at earlier stages, thus preventing morbidity and scarring complications.
Preoperative imaging work-upThe surgical goal of HS surgery is to remove all the inflamed tissue to prevent further relapses.12 Preoperative imaging modalities such as magnetic resonance, infrared thermography, optical coherence tomography, or reflectance confocal microscopy have been studied.13 Currently, among them, color Doppler ultrasound is considered crucial for evaluating patients with HS.14,15 This noninvasive and widely available imaging modality provides high-resolution visualization of subclinical millimetric lesions located in the dermis and subcutaneous tissue.13
In a multicenter study involving 143 HS patients, Martorell et al. showed that high-frequency ultrasound (HFUS), using a linear probe with a high-frequency image of 18MHz, significantly (p<0.01) upstaged the clinical disease classification, modifying its therapeutic approach.16 Ultrasound surgical margin delimitation significantly increased the excised area by >3.5cm2 (p=0.004) and reduced the rate of recurrence from 30% down to 10% (p=0.10) vs clinical delimitation.17 Preoperative ultrasound helps preserve neighboring vasculonervous structures and spares healthy tissue, facilitating surgical excision. It also helps other surgical approaches such as deroofing of sinus tracts by identifying additional tunnels and ensuring their complete removal.18
Some ultrasound signs have been defined to predict the lack of response to adalimumab, allowing for more adequate and timely preoperative planning. These signs include the presence of wall tunnel fibrosis,19,20 vascularization,20 and pseudoepithelization of the tunnel walls, described as the railway sign.21 Recently, quantitative shear wave elastography, a new non-invasive imaging modality that measures tissue elasticity or stiffness of tissues, has shown promising results in evaluating HS fibrosis.22 However, its exact role in the preoperative setting has yet to be defined.
Local anesthesiaMost HS surgical procedures can be performed under local anesthesia, tumescent local anesthesia (TLA) being widely used. TLA involves subdermal injection of large volumes (typically>100mL) of highly diluted local anesthetics, often lidocaine buffered with sodium bicarbonate.23,24 The advantages of this technique include reduced intra- and postoperative bleeding—also reduced by the routine use of epinephrine along with the anesthetic agent—postoperative swelling, pain, or risk of infection,25,26 but its use in HS surgery has not been widely reported.
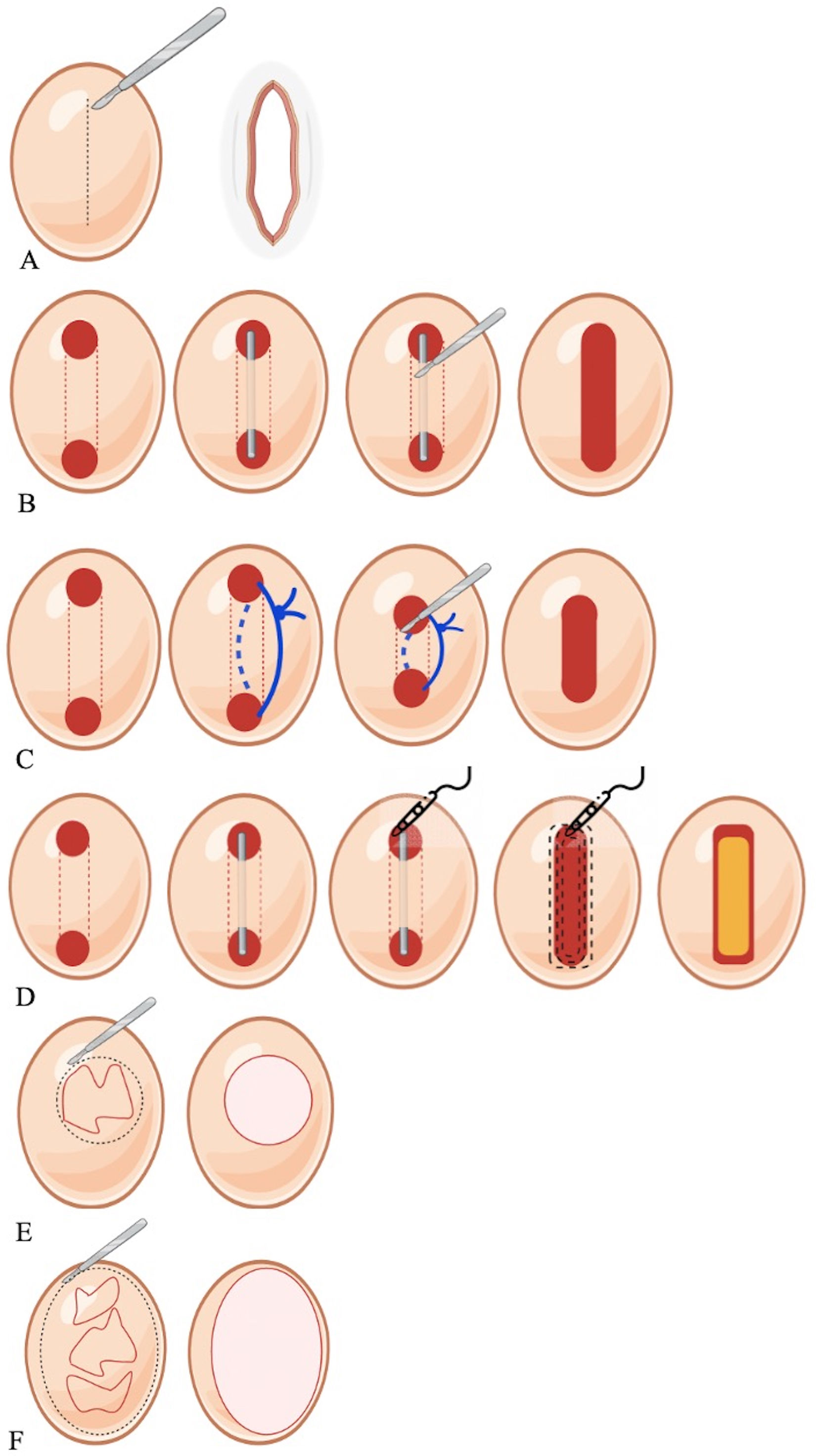
Which type of surgery should be selected for each patient?HS surgery consists of 2 main parts: incision and removal of the involved tissue (Fig. 2) and closing or reconstruction of the surgical defect. The choice of surgical technique depends on factors such as lesion extent and severity, or the anatomical area affected,27,28 as well as the patients’ preferences. Rates of recurrence vary for each technique, being generally higher when not all affected tissue is resected with a radical margin, ranging from 26% in partial excisions down to 5% in regional excisions (p<0.01).29,30 Since few randomized controlled trials have compared different techniques, the quality of evidence is low,31,32 making treatment choice a significant challenge.
- 1.
Surgical incision and drainage
This treatment is spared for acute lesions in the form of painful fluctuating abscesses. It typically has a temporary effect, with recurrence rates close to 100%, and should generally be avoided unless symptomatic pain management is required.28 Some authors prefer deroofing, achieving similar symptomatic relief with fewer recurrences.28
- 2.
Deroofing
Deroofing involves removing the “roof” of a nodule, abscess or sinus tract.33 These structures, including epithelialized tunnels, contain active inflammatory tissue, and their removal by this limited surgery reduces the inflammatory burden while sparing the adjacent healthy tissue, leaving a cosmetically acceptable scar.33 Ideal candidates for this treatment are patients with limited Hurley I or II stage disease located in the axillar or inguinal area.18 Lesions located in the perianal or perineal area should be previously investigated to rule out the presence of colorectal or urogenital fistulae.18
Lesions are clinically identified by visualization and palpation, and preferably delimited with HFUS. Under local anesthesia, a blunt probe inserted in the sinus openings guides the removal of the “roof” of the lesion with a scalpel, scissors or an electrosurgical loop, leaving the floor of the lesion exposed to healing by secondary intention.33 In patients with Hurley stages I or II, the recurrence-free rate of deroofing is 83% after a median follow-up of 34 months, with a median satisfaction rate of 8 on a 0–10 scale.33 Ultrasound examination can identify hypoechoic inflammatory collections or occult epithelialized tunnels for proper treatment.18 CO2 lasers are used for deep, large, interconnected or recurrent cavities.11 For small abscesses or tunnels, a mini-deroofing technique using a punch biopsy can be used as an in-office procedure.34 Setons—nonabsorbent silicone loops—introduced through the openings of a sinus tract and secured with surgical knots, can decrease inflammation and drainage from the sinus tract, facilitate epithelization, and induce gradual migration of the sinus tract toward the skin surface, resulting in spontaneous deroofing and resolution of the fistulae, or leading to shallower fistulae more accessible to surgical intervention.35 In a retrospective case series of 34 sinus tracts (27 Hurley II and Hurley III stage HS patients), seton placement was associated with a significant (p<0.001) reduction in pain and sinus tract depth.36 In 16 cases no further treatment was required after seton removal, 3 setons were spontaneously extruded, 12 required deroofing, and 3 required excision of the fistulae. At the 16-week follow-up, only 2 recurrences (6%) (both after radical excision) were detected.36 Therefore, setons are promising in the surgical management of HS, but further studies are needed to confirm their safety and efficacy profile.
- 3.
Skin-tissue-sparing excision with electrosurgical peeling (STEEP)
Developed for treating HS lesions in Hurley stages II-III, STEEP removes all the affected tissue while sparing as much healthy tissue as possible to prevent any contractures, which is of particular importance in skin folds, and facilitates wound closure, which is more difficult when a regional excision has been performed.37 Performed under general anesthesia, STEEP starts by locating the inflammatory nodules and fibrosis through palpation or, preferably, via perioperative imaging modalities. Sinus tracts are probed, their extent is assessed, and sinus roof is subject to electrosurgical incision with a wire loop tip, as in the deroofing technique. Lesional tissue and fibrosis are removed through successive tangential electrosurgical transections, while sparing epithelialized sinus floors and subcutaneous fat as much as possible. In this technique, an attempt is made to preserve the floor of non-inflammed tunnels, while inflammed tunnels are subjected to a complete curetting procedure. Wound margins are meticulously checked for residual sinus tracts and injected with triamcinolone acetonide and bupivacaine to prevent the formation of excessive granulation tissue. The resulting wounds are left open for secondary intention healing.37 This technique includes the complete removal of fibrotic tissue, theoretically leading to a low rate of recurrence, yet reports indicate up to a 50% rate of recurrence within the initial year.38 Nevertheless, the existing evidence is limited, predominantly consisting of case series lacking long-term follow-up data.28 Therefore, STEEP should be spared for solitary or recurrent limited lesions.32
- 4.
Lesional excision
Lesional excision—also called limited excision—involves removal restricted to the tissue involved. Lesional excision typically consists of removing each individual lesion separately while ensuring adequate margins.27,39 Primarily spared for solitary lesions, or a small number of lesions confined to limited areas,32 this technique was initially performed under local anesthesia in Hurley I or II stage patients with recurrent lesions usually smaller than the size of a palm of the hand, achieving clear margins and allowing primary closure.40 In a series of 92 lesional excisions, Van Rappard et al. reported a rate of recurrence of 23% after a mean 10 months, and a mean recurrence-free follow-up of 27 months.40 Although complications were mild—including bleeding and infection—20 cases (22%) of suture dehiscence were reported.40
- 5.
Regional excision
Regional excision—formerly referred to as wide excision—involves removing all lesions within the affected area in a single block, including nodules, non-inflamed tunnels, scar tissue, and adjacent healthy tissue.39,41 Although excision margins may vary, a margin of up to 1cm in the subcutaneous tissue or extending to the fascia has been suggested.41 Since these surgical procedures may require wide resections involving adjacent tissues, these procedures usually require collaboration with other specialists such as urologists, general surgeons, or plastic surgeons. Rates of recurrence following regional excision go from 5% up to 18%,29,41 the lowest among surgical procedures. For this reason, it has traditionally been considered the surgical treatment of choice for HS, especially in severe cases.27,37 Regional excision is currently recommended for Hurley III HS to prevent further recurrences.32
Reconstruction options after regional excisionAlthough regional excision is an optimal technique for managing HS, the resultant large wound areas may lead to contractures and prolonged healing times. Various methods exist for managing the defect, each with different recurrence rates and complications, requiring adequate knowledge for appropriate selection.42,43
- 1.
Primary suture
Primary closure can be considered for minor excisions surrounded by lax skin, especially in small-sized lesions and lower-grade HS cases.27,44 Although it may be the closure technique preferred by patients,27 it is associated with the highest rates of recurrence among closure techniques (70%).45 Loose wound closure is recommended, allowing for drainage of exudate and reducing the risk of seroma and infection.44 Wound dehiscence, scarring and contractures can also occur, so closing under tension is not advisable.24
- 2.
Secondary intention healing
It consists of allowing wound closure through the natural process of granulation, shrinkage, and epithelialization.44 It is the method of choice for techniques such as deroofing or STEEP,33,37 with variable mean wound closure time depending on the wound area from 14 up to 53 days.33,38 Furthermore, it is also useful for regional excisions up to 140cm2, particularly in the anogenital, trunk or axillary area,24 with rates of recurrence close to 12%.46 In these cases, it is associated with cosmetic scars of a smaller size than the initial defect, without additional scarring of donor sites, and avoiding significant loss of mobility.11,24,44 However, secondary intention healing requires long healing times and a need for meticulous wound care and dressing changes.44 There is also a risk of wound contracture, especially with larger excisions.44
- 3.
Split-thickness skin grafts (STSG)
STSG, harvested from thighs or buttocks and expanded in a 3:1 ratio with multiple incisions, are widely used for large wounds, with minimal risk of complications. Traditionally, grafts were applied to granulation tissue after prolonged wound conditioning.24,44 However, grafting has good hemostatic properties and can be performed in a single surgical act immediately after excision, especially if negative pressure devices are used to promote granulation tissue.47 STSG provide acceptable outcomes, particularly in areas such as the armpits and buttocks, where graft contraction or color changes do not cause major functional or esthetic problems.24,44 Good results have been reported with a 2-stage approach, first applying an artificial dermis graft and then a normal skin graft.48 This approach is especially useful in cases where wide and deep excisions have to be performed, avoiding depression deformities and lack of tissue flexibility.44,48
- 4.
Skin flaps
Reconstruction of defects with flaps offers superior quality of skin closure and may prevent contractures and severe scarring vs other reconstructive techniques. However, flaps pose challenges such as a more complex and invasive harvesting procedures and a higher risk of complications, such as tissue necrosis or bleeding.44 Since local recurrences can occur if mobilized skin is affected by HS, flap procedures can only be conducted when clean margins can be ensured.44 Flap procedures are essential for covering vital anatomical structures such as exposed neurovascular bundles. Although various flap techniques have been described depending on the anatomical site,49 no gold standard technique has been recognized to this date.50
ConclusionsEarly surgical treatment of HS, along with medical therapy, may represent the standard of choice for HS. Given the higher rate of recurrence associated with leaving lesional tissue remnants, deroofing, STEEP, or partial excisions may be considered procedures of choice for Hurley stages I–II, whereas regional excisions are preferred for more severe Hurley II–III disease. Reconstruction techniques would vary, primarily depending on the extent of the defect and the area involved. In all cases, good preoperative planning and delimitation with imaging modalities—preferably intra- or perioperative—facilitate complete removal of the tissue involved, preserve healthy structures, reduce recurrences and allow us to benefit from the surgical window of opportunity.
Conflicts of interestLM declared no conflicts of interest whatsoever. EV has perceived consultancy/speaker's fees from and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Bayer, Biofrontera, Boehringer Ingelheim, Celgene, Galderma, Gebro, Isdin, Janssen, Leo-Pharma, Lilly, Merck-Serono, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, UCB. L. Puig has perceived consultancy/speaker's fees from and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius-Kabi, Horizon (DSMB), J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, STADA, Sun-Pharma, Takeda, and UCB.