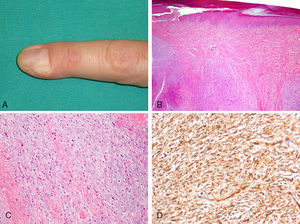
We report on the case of a 40-year-old patient, with no relevant medical history, who consulted for a periungual tumor on the second finger of the left hand. The lesion, which was asymptomatic, had appeared 2 years earlier, and had shown slow, progressive growth. The physical examination showed a 1-cm diameter tumor with poorly defined borders that was not attached to the deep layers (Fig. 1A). The lesion was firm and slightly painful on palpation. Four years earlier, a similar lesion—diagnosed as periungual fibroma—had been excised from the same location.
A, Subcutaneous nodule on the second finger of the left hand, causing partial deformation of the nail matrix. B, Dermal tumor composed of alternating fibromyxoid and myxoid areas. C, Spindle-shaped cells embedded in a myxoid stroma. Also visible are numerous mast cells with a round nucleus and abundant cytoplasm (hematoxylin–eosin, original magnification ×100). D, Diffuse cytoplasmic positivity for CD34 in neoplastic cells (hematoxylin–eosin, original magnification ×200).
Histologic examination of the current lesion, removed by simple excision with curettage of the base, showed an epidermis with mild orthokeratotic hyperkeratosis and a poorly circumscribed dermal neoplasm (Fig. 1B) formed by a population of spindle cells embedded in loose stroma composed of alternating myxoid and fibromyxoid areas of identical cell density, without a clearly defined architecture (Fig. 1C). There were also numerous mast cells that stained positive for CD117. Alcian blue staining showed a predominance of acid mucopolysaccharides in less fibrous areas. There was no evidence of nuclear atypia or mitosis. Neoplastic cells were diffusely and strongly positive for CD34 (Fig. 1D) and CD99 throughout the thickness of the tumor, and there was a low Ki-67 index (<5%). Staining for epithelial membrane antigen (EMA) and S100 protein was negative. Based on these findings, a diagnosis of superficial acral fibromyxoma (SAF) was established. Because the lateral and deep borders of the resection specimen were affected, extension of the surgical field was considered, but the patient refused a second operation. He has attended regular follow-up visits, and at the time of writing, 6 months later, he remains asymptomatic. Magnetic resonance imaging at the 4-month follow-up showed no evidence of residual tumor.
SAF is a neoplasm that was first described in 2001 by Fetsch et al.,1 who reported on a series of 37 cases after conducting a review of 280 fibrohistiocytic tumors of distal extremities diagnosed over 3 decades. The tumors evaluated included fibroma, fibromyxoma, myxoma, myxolipoma, dermatofibroma, fibrous histiocytoma, and angiofibroma. Since then, several isolated case reports and series have been published.2–6 SAF is a benign lesion1,2 and there have been no reports of malignant transformation or metastasis. The few cases of recurrence that have been reported have been associated with incomplete resection.3,4 The literature contains reports of approximately 100 cases of SAF and several authors consider that this tumor is underdiagnosed.
Clinically, SAF is more common in men than in women, with a ratio of 2 to 1, and it has been described in patients aged between 14 and 75 years (median, 46 years).1 It tends to have a firm consistency and generally grows slowly and painlessly, explaining why many patients delay visiting their physician. It primarily affects, by order of frequency, the toes (mainly the big toe), the fingers, and more rarely the palms and the soles.1,3,4 Nail involvement is seen in 50% of cases, with hyperkeratosis or onycholysis accompanied occasionally by pain on compression. Erosion of the underlying bone is rare but it has been reported.7
Histologically, SAF is a well-circumscribed—but not encapsulated—dermal or subcutaneous tumor with increased vascularization1–5; these findings contrast with those observed in our patient. There have been some reports of multinucleated stromal cells, areas of necrosis, lipomatous areas, and epidermal signs of viral infection.1–5,8 Detection of viral infection led to the suggestion that human papillomavirus might be involved in the etiopathology of SAF.4 Immunoreactivity to CD34 is common, but there are tumors that test negative for this marker.9 CD10, CD99, EMA, and nestin immunoreactivity are also common. Given the immunoreactivity of these markers, several authors have postulated that mesenchymal stem cells residing in distal extremities might be the origin of SAF.1,8,10 Immunohistochemical staining for S100 protein, actin, desmin, cytokeratin, apolipoprotein D, and HMB45 is almost always negative.1,2,4
On detection of CD34 immunoreactivity, it is necessary to rule out other entities that stain positive for this marker, in particular myxoid variants of dermatofibrosarcoma protuberans (DFSP) (Table 1). It was traditionally considered that myxoid DFSP was more common than other variants of this tumor in distal extremities, but this could be because many cases diagnosed as myxoid DFSP were actually SAF. Myxoid DFSP expresses apolipoprotein D and presents with a storiform pattern and characteristic subcutaneous infiltration. Diagnosis is confirmed by detection of the chromosomal translocation t(17;22). The differential diagnosis should include benign myxoid lesions with spindle-shaped cells (myxoid neurofibroma, superficial angiomyxoma, mucous cyst), malignant myxoid lesions with spindle-shaped cells (myxoid DFSPM, acral myxoinflammatory fibroblastic sarcoma, low-grade fibromyxoid sarcoma, myxofibrosarcoma), and other acral neoplasms such as sclerosing perineurioma, periungual fibroma, digital fibrokeratoma, and cellular digital fibroma. This last entity is composed of spindle-shaped CD34-immunoreactive cells and can be distinguished from SAF as it is less myxoid and more cellular, although several authors have postulated that they are the same entity.11
Spindle-Cell Tumors That Express CD34.
| Tumor by Frequency of CD34 Positivity | Characteristic Findings | Immunohistochemistry |
| Very frequent | ||
| Dermatofibrosarcoma protuberans | Chromosomal translocation t(17;22)Storiform pattern and fingerlike subcutaneous infiltration | Positive for apolipoprotein D, actin, and vimentinNegative for S100 protein, HMB5, cytokeratin, and factor XIIIa |
| Solitary fibrous tumor | Very rare benign tumor of the skin, generally located on the head or neck. Highly variable histologic findings | Positive for CD99 and vimentinNegative for S100 and muscle markers |
| Sclerotic fibroma | Similar to solitary fibrous tumor | |
| Nerve sheath tumors (neurofibroma, neuroma) | Wavy nuclei | Positive for S100 protein |
| Spindle-cell lipoma | Positive for S100 protein | |
| Superficial acral fibromyxoma | Fibromyxoid areas, increased vascularization, and presence of mast cells | EMA, CD99, CD10, and nestin positivityNegative for S100, apolipoprotein D, cytokeratin, vimentin, and desmin |
| Cellular digital fibroma | Similar to superficial acral fibromyxomaLess myxoid and more cellular | Similar to superficial acral fibromyxoma |
| Variable/occasional | ||
| Epithelioid sarcoma | Epithelioid nodules or groups of cells surrounding a central necrotic area | Positive for cytokeratin, EMA, and vimentin |
| Acral myxoinflammatory fibroblastic sarcoma | Infiltrative growth pattern, considerable inflammatory component, myxoid or hyaline stroma with 3 populations of tumor cells (multinucleated Reed–Sternberg-like cells, epitheloid cells, and spindle-shaped cells). | Positive for vimentin and occasionally for CD34 and CD68Negative for EMA and S100 |
| Low-grade myxofibrosarcoma | Significant pleomorphism, numerous atypical mitoses, prominent curvilinear capillaries | Positive for vimentin and actinOccasionally positive for CD34Negative for desmin and EMA |
| Angiosarcoma | Vascular lumens with an infiltrative pattern, blood extravasation, and hemosiderin deposits | Positive for blood and lymph vessel markers (F-VIII-RA, D2-40, CD31) |
| Dermatofibroma | Rarely positive for CD34Positive for factor XIIIa | |
The treatment of choice for SAF is surgical resection with tumor-free margins.1,3 Because SAF can recur, patients should be closely monitored after surgery, particularly when, like our patient, they have involved resection margins.1,3
In conclusion, dermatologists and dermatopathologists should include SAF in the differential diagnosis of fibrohistiocytic tumors of distal extremities. Early diagnosis and complete resection are key to preventing recurrence.
Please cite this article as. Messeguer F, et al. Fibromixoma acral superficial, un tumor periungueal CD34 positivo. Actas Dermosifiliogr. 2012;103:67–9.