One of the main goals of all skin cancer prevention campaigns is to protect children from ultraviolet radiation. However, little is known about how sun exposure risks differ between adults and children or about how these risks are best managed. Children's skin is more susceptible to sun damage for a number of reasons, including certain anatomical and functional aspects in children under 2 years of age and habits that predispose to greater sun exposure during the first 2 decades of life. Oil-based emulsions containing inorganic filters appear to be safest sunscreens for children, although the addition of certain organic filters is necessary to achieve a sun protection factor of 50. Oxybenzone, and probably also octocrylene, should be avoided in sunscreens for children. Sunscreen use should be part of an overall sun protection strategy that includes avoidance of exposure to midday sun and the use of protective clothing and hats.
The above considerations justify the implementation of primary prevention campaigns focused on sun protection education for children and the continuation of basic and epidemiological research into specific sun protection strategies and sunscreens for each age group.
Proteger a los niños de la radiación ultravioleta es uno de los principales objetivos de todas las campañas de prevención del cáncer cutáneo. Sin embargo, el conocimiento acerca de las diferencias en riesgos derivados de la fotoexposición con respecto a los adultos y de las estrategias idóneas para afrontarlos son escasos. Entre los factores que favorecen una mayor susceptibilidad de la piel infantil se encuentran ciertos condicionantes anatómicos y funcionales en los niños por debajo de los 2 años de edad y hábitos de mayor exposición en las 2 primeras décadas de la vida. Los filtros en forma de emulsión en aceite con principios activos inorgánicos parecen ser los más seguros para los niños, aunque se requiere la adición de algunos filtros orgánicos para obtener un SPF 50. La oxibenzona y probablemente el octocrileno son filtros que deberían evitarse en los fotoprotectores pediátricos. El uso de fotoprotectores debe ser parte de una estrategia fotoprotectora basada en evitar la exposición solar en las horas del mediodía y usar ropas y gorros.
Todo ello justifica la implementación de campañas de prevención primaria que eduquen a los niños en hábitos de fotoprotección, y continuar la investigación básica y epidemiológica en la búsqueda de estrategias y fotoprotectores concretos para cada edad.
The sun allows the existence of life on earth. It provides a feeling of well-being and controls our biological rhythms. It is also fundamental for the synthesis of vitamin D. However, solar radiation is also known to have harmful effects in both the short term (erythema, immunosuppression) and the long term (photocarcinogenesis, actinic damage). In order to minimize these adverse effects, a series of measures, known broadly as sun protection, have been developed. Sun protection measures are considered to be appropriate for the entire sun-exposed population. It has been suggested, however, that children may be especially susceptible to the harmful effects of solar radiation. These considerations, as well as social demand, have given rise to the development of sun protection product lines designed exclusively for children and the design of primary prevention strategies targeting children.
Although it may seem bold, it is fair to ask whether it really makes sense to consider children to be a differentiated target for sun protection measures. In other words, is there scientific evidence to justify distinguishing sun protection strategies for children from those intended for the rest of the population? A specific sun protection strategy for children would be reasonable if, for physiological reasons related to age or habits, childhood were intrinsically an additional risk factor for adverse effects of solar radiation. Children should also be considered separately if the components of sun protection strategies—for example, excipients contained in sunscreens or even the UV filters themselves—carry an additional risk in childhood because of the direct risks (allergic contact dermatitis, carcinogenesis) or indirect risks (e.g., hypovitaminosis D due to inadequate sun exposure) associated with these substances.
A second issue is also important: even if we establish that children are a differentiated group that requires a specific sun protection approach, we must determine whether the available strategies and technical possibilities are properly implemented.
Is the Risk Associated With Sun Exposure Greater in Children Than in Adults?Risks Related to Sun Exposure HabitsThe data on sun exposure in children are generally limited and mostly come from interviews with selected, voluntary groups. There is a tendency to consider that sun exposure time is greater in childhood than in adulthood. If this is true, much of the sun exposure—and, consequently, the risk—that an individual accumulates over the course of his or her lifetime occurs during childhood. After reviewing data on 345 schoolchildren, Wright and Reeder1 estimated that the mean daily sun exposure time was 2.3 hours and determined that exposure was greater on school days than on weekends. They therefore concluded that sun protection should be promoted in schools.
The erythema dose associated with this number of sun exposure hours is incredibly difficult to establish and, moreover, varies greatly as a function of latitude and climate conditions. It is generally considered that 25% to 50% of the total erythema dose that a person receives before the age of 60 years occurs during childhood (in accordance with the Convention on the Rights of the Child, adopted in 1990, a child is defined as a person under the age of 18 years).2,3 It therefore appears that many individuals are most exposed to solar radiation during childhood and that the promotion of sun protection in children is justified.
Risks Related to Structural Differences Between Children's Skin and Adult SkinAnother additional risk factor for children's skin could be related to the fact that children may be more susceptible than adults to damage caused by sunlight or that children's defense mechanisms may be less efficient than those of adults. The assumption that children's skin is more sensitive or susceptible to sun damage than that of adults appears to be justified in children under the age of 2 years.4Table 1 highlights the structural differences between infant skin and adult skin.5 In children, the stratum corneum and the epidermis as a whole are thinner and corneocytes and granular cells are smaller and more numerous, suggesting more rapid cell turnover. In addition, the ratio of lipids to proteins is lower, as is the concentration of melanin. Diffuse reflectance spectroscopy studies have shown that the skin of children under the age of 12 months has a lower concentration of melanin than that of children between the ages of 16 and 24 months.6
Differences Between Skin Structure in Infants and Adults42
| Skin Structures | Infant Skin | Adult Skin |
| Epidermis | ||
| Corneocytes | Smaller | Larger |
| Granular cells | Smaller | Larger |
| Depth of dermatoglyphics | Similar to in adults | |
| Melanin | Less | More |
| Dermis | ||
| Dermal papillae (density, size, and morphology) | More uniform | Less uniform |
| Transition between papillary and reticular dermis | Absent | Present |
| Skin characteristics | ||
| Concentration of natural moisturizing factor | Lower | Higher |
| pH | Higher (only in newborns) | Lower |
| Sebaceous secretion | Less | More |
| Water content in stratum corneum | Higher | Lower |
| Collagen fiber density | Lower | Higher (in young adults) |
| Skin functions | ||
| Water absorption | Higher | Lower |
| Water desorption | Higher | Lower |
| Barrier function | Competent | Competent |
| Transepidermal water loss | Higher | Lower |
In children older than 24 months, the thickness of the skin is similar to that of adults. However, because of the special anatomic structure of children's skin, the most superficial part of the dermal papillae is more exposed to solar radiation. The stem cells in the basal layer are therefore exposed to higher doses of UV radiation, thereby favoring the initiation step of nonmelanocytic skin cancer7 (Table 1).
Nevertheless, these differences in skin structure do not necessarily have an impact on certain photobiologic aspects, such as erythema. It has been shown that there are no differences in minimal erythema dose between adults and children under the age of 15 years.8
Key Point
The skin of children under the age of 2 years has anatomical and functional characteristics that make it more susceptible to UV radiation damage than adult skin.
Although there are some structural differences between children's skin and adult skin, there are no differences in minimal erythema dose.
Given that tanning is initiated by the activation of p53 when DNA damage occurs and that incidental sun exposure induces tanning in children as young as 1 year of age,9 we can conclude that the damage caused by actinic radiation begins in the first years of life. With regard to melanogenesis as a defense mechanism against solar radiation, it is fair to ask whether there are functional differences between children and adults. Pigmentation occurs in children as a response to solar radiation from the age of 6 months but is less pronounced between 6 and 12 months of age than between 16 and 24 months of age, illustrating the variations in melanogenesis in relation to age.10
The development of melanocytic nevi is considered a sign of actinic damage and a marker of melanoma risk. In an epidemiologic study carried out in Australia, Whiteman et al.11 found that the presence of more than 10 melanocytic nevi at least 5mm in diameter increased the risk of developing melanoma in childhood by a factor of 9.9.
One international epidemiologic study that compared children aged 8 or 9 years of the same ethnicity found that the number of nevi was up to 7 times greater in children living at latitudes that receive more solar radiation (Australia as opposed to Great Britain).12 Another study found an epidemiologic association between nevus prevalence and the number of site-specific sunburn episodes.13 Some authors have suggested that intermittent episodes of excessive sun exposure during outdoor sports or vacation activities play a greater role than cumulative sun exposure in increasing the risk of melanocytic nevi.14,15 However, this hypothesis has not been conclusively demonstrated.
Melanocytic nevi in children differ from those in adults in terms of epidemiology, morphology, genetics, and associated risk of melanoma. It has been hypothesized that these differences are present because nevi are derived from melanocytes at different stages of maturation.16
Actinic Radiation in Childhood and Risk of Skin Cancer in AdulthoodMelanomaWithout a doubt, one of the greatest risks apparently associated with sun exposure is the possibility of increased risk of melanoma. Overall, the risk of melanoma in childhood is low. Only 2% to 3% of all cases of melanoma occur in children.17 However, this risk increases over time: 85% of cases of melanoma in patients under the age of 20 years occur between the ages of 15 and 19 years.18
It is likely that genetic predisposition plays a much greater role in melanomas that develop in the first years of life, whereas solar radiation could play a greater role in causing melanomas that develop during adolescence. In a case-control study of melanoma in adolescents, the number of sunburn episodes was higher in the cases than in the controls.19 Other data also support this hypothesis. An association has been found between higher incidence of melanoma and the latitude of residence during childhood, suggesting that solar radiation may be involved in a similar way as in adulthood.20 Some data on immigrant populations have shown that the risk of melanoma in individuals who immigrated in the first years of life is equal to the risk in the native population; this seems to support the notion that solar radiation in the first years of life plays a role in the incidence of melanoma.21
In a case-control study conducted in 1990, it was suggested that the risk of melanoma associated with solar radiation in adults is influenced by the amount of sun exposure during childhood.22 However, it is important to take into account numerous confounding factors, including the amount of radiation received in adulthood, personal sun exposure habits, and the number of sunburn episodes.
One factor that may influence the effect that early sun exposure has on the development of melanoma in adulthood is the nature of the genetic damage induced by radiation during childhood.23 One study found that BRAF mutations were more common in patients with primary invasive melanoma with a mean age of 47 years at diagnosis and a history of intense sun exposure in the first years of life, whereas NRAS mutations were more common in patients with a history of greater sun exposure between the ages of 50 and 60 years and a mean age at diagnosis of 62 years.24 In agreement with these data, another study found that BRAF mutations were more common than NRAS mutations in acquired melanocytic nevi in sun-exposed areas in children, whereas most nevi present from birth presented NRAS mutations. However, the study found that BRAF mutations were more common in nevi acquired later in life that had histologic features consistent with congenital nevi.25
Nonmelanocytic Skin CancerThe prevalence of nonmelanoma skin cancer in children is practically negligible, except in patients with DNA repair-deficiency disorders such as xeroderma pigmentosum and basal cell nevus syndrome.25 Although sun exposure appears to play an important role in these cases, the importance of cumulative childhood solar radiation in the development of nonmelanoma skin cancer during adulthood is unknown. A recent study found that childhood sun exposure plays a greater role in the development of squamous cell carcinoma than in basal cell carcinoma.26
Risks Associated With Skin PhototypeThe risk of most forms of melanoma in adolescence is associated with the genes that control skin pigmentation and the propensity to develop melanocytic nevi. Large numbers of nevi and freckles, red hair, blue eyes, inability to tan, and family history are the main determinants of melanoma risk in adolescents.27 This phototype-related risk has a role in the clear differences in skin cancer risk between children and adults who emigrate from high to low latitudes.3
In a study carried out in Colorado (United States) on the development of melanocytic nevi in children aged 3 to 8 years, non-Hispanic white children developed more melanocytic nevi (around 4-6 new nevi per year) than children of other racial groups, and the mean number of new nevi in chronically exposed body sites was higher in boys than in girls.28 If the number of melanocytic nevi is a risk factor for melanoma, these children could face a higher risk of melanoma in direct relation to their sun exposure in childhood.29
Risks Associated With ImmunosuppressionVarious studies—conducted in laboratory models and adults—have shown that UV radiation suppresses both primary immune responses and memory T cells that respond to a wide variety of antigens.30 In young children, however, it is presumed that the effects of UV radiation can modify not only present but also future immune responses. In newborn mice, UV radiation is associated with decreased cytokine synthesis and depletion of Langerhans cells, diminishing these cells’ capacity to capture antigens and ability to induce T-cell proliferation. It has also been shown that UV radiation suppresses the helper T cell–type response and favors an increase in the number of regulatory T cells in the lymph nodes.31
The aforementioned effects of UV radiation could favor antigen-specific immunosuppression, increasing the risk of skin cancer in the future. Experimental observation of how, in laboratory models, a single dose of UV radiation can cause cancer in neonates but not in adults illustrates how much greater the impact of actinic damage can be in an immature immune system.32
In the opinion of some authors, even the effectiveness of vaccination could be influenced by the immunomodulatory effects of UV radiation.33
However, not all effects of UV radiation on the immune system of children are harmful. UV radiation also provides protection against the development of photodermatoses such as polymorphous light eruption and T cell–mediated autoimmune diseases such as multiple sclerosis and asthma.
Is Sun Protection Associated With Specific Risks in Childhood?Risk of Melanocytic Nevi and Melanoma in Sunscreen UsersSunscreen use is the most common method of sun protection in both the general population and children.34 The authors of a study published in 1998 found that sunscreen use was associated with the development of melanocytic nevi in white European children and hypothesized that sunscreen use may encourage longer sun exposure.35 This observation seemed to suggest that sunscreen use can have negative effects insofar as it leads to greater sun exposure. However, later research has suggested that the use of broad-spectrum, high sun protection factor (SPF) sunscreens has the opposite effect, namely, a reduction in the number of new melanocytic nevi in children, especially those with freckles.36 A similar controversy has arisen with regard to the relationship between sunscreen use and melanoma risk. Although the available data are inevitably indirect and subject to considerable bias, the one meta-analysis that has been published on this subject concluded that there is no evidence of an association between topical sunscreen use and increased risk of melanoma.37 In fact, some authors have argued that regular sunscreen use may prevent melanoma.38 A recent meta-analysis concluded that there is currently no evidence that sunscreens protect against the development of melanocytic nevi in children.39
Risks Associated With Topical and Systemic Adverse Effects of SunscreensThe molecules contained in sunscreens are generally considered to be safe. The molecules in the latest generation of sunscreens, in particular, are relatively large and mostly do not permeate the epidermis, limiting the likelihood of absorption or interaction with the immune system.40
However, it should be remembered that the stratum corneum continues to develop throughout early childhood.41 During the first year of life, infant skin is more hydrated and more permeable to water and has a higher concentration of natural moisturizing factor.42 Given these circumstances, it is especially important to protect children against the risk of absorption and adverse reactions to sunscreens.
Oxybenzone (benzophenone-3) is a broad-spectrum UV filter. It has been shown to penetrate human skin in vitro and has also been found in urine following topical application. In a population-based study, oxybenzone was found in the urine of 97% of the population aged 6 years and over.43 However, oxybenzone penetration has not been shown to interfere with the hypothalamic-pituitary-gonadal axis in adults.44 Because various UV filters, including oxybenzone, have been detected in breast milk in quantities proportional to the amount of sunscreen applied to the mother's skin, limited use of sunscreen in pregnant women and infants has been recommended. To date, no studies have demonstrated the toxicity of systemic absorption of sunscreen ingredients.41
Octocrylene is an ester formed by the condensation of diphenylcyanoacrylate with 2-ethylhexanol, which belongs to the cinnamate family. Introduced approximately a decade ago, octocrylene is a filter that absorbs both UV-B and near–UV-A radiation. It also plays an important role as a stabilizer of many other filters (especially cinnamates and butyl-dibenzoylmethane), increasing their efficiency and making them more water-resistant.
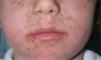
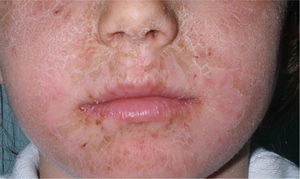
Because of its advantageous physical properties, octocrylene is becoming increasingly common and is being used at higher concentrations. Today, more than 300 sunscreens commercially available in Spain contain octocrylene. It is considered overall to be a safe substance, but allergic and photoallergic reactions have been reported in recent years, even in small case series of children (Fig. 1). In adults, octocrylene is often associated with photoallergy, frequently because of cross-reaction with ketoprofen, but in children the most common manifestation is allergic contact dermatitis; octocrylene is almost always of present relevance.45
Retinyl palmitate is found in many cosmetic products and it is also used as a food additive. It has recently been suggested that this compound plays a role in photocarcinogenesis by producing excessive reactive oxygen species. Retinol (vitamin A), an essential nutrient that has many biological functions, accumulates in the skin in the form of retinyl palmitate. Studies in mice have shown that retinyl palmitate increases the photocarcinogenic activity of UV-B radiation.46 However, after more than 50 years of use in humans, there is no clinical evidence of such an effect in humans.47
In order to minimize all of the aforementioned risks in children, the use of sunscreens containing inorganic filters has been widely encouraged. Inorganic filters such as zinc oxide and titanium dioxide have the advantage of being highly photostable and nonallergenic. However, sunscreens with inorganic filters are less aesthetically appealing than those which contain organic filters. Technological advances have made it possible to develop increasingly small molecules (20-50 nm as opposed to 200-300 nm in earlier formulations). Sunscreens that are transparent to visible light, containing molecules smaller than 0.2 μm, have also been developed.48 The application of nanotechnology has made sunscreens containing organic filters more transparent and, therefore, more aesthetically appealing. However, the smaller particle size raises questions about whether the molecules might be absorbed through the skin and, therefore, about the toxicity of these sunscreens. A recent study of a sunscreen containing nanosized titanium dioxide applied to the skin of animals showed that these nanoparticles remained as aggregates in the superficial layers of the epidermis, both in intact skin and in skin irradiated by a solar simulator.49 For the moment there is no evidence that these particles are absorbed at higher rates or that they have any potential cytotoxic or genotoxic effects. However, there is no evidence to support—or advise against—their use in infants and children. Titanium dioxide is widely used as a whitening agent in toothpaste and skim milk, and zinc oxide is a common ingredient in barrier creams for infants and in cereals. The widespread use of these compounds without any problems having been detected supports the notion that they are safe to use in infants and children.41
Under baseline conditions, titanium dioxide and zinc oxide are unable to penetrate beyond the stratum corneum, but doubts could be raised about the use of these substances in individuals in whom the stratum corneum is not intact.50 No studies carried out to date in human or animal skin have shown that the use of these substances is unsafe, even in individuals with damaged skin (for example, with atopic dermatitis).51
Because sunscreens are often used in combination with insect repellants during the months of greatest sun exposure, it is important to remember that sunscreens can increase the absorption of repellants containing N,N-diethyl-meta-toluamide, especially when the repellant is applied first. Likewise, the use of products that contain both sunscreen and insect repellant is not recommended.41 Moreover, sunscreens must be reapplied but insect repellent should not be, in order to avoid toxicity due to percutaneous absorption.52
Key Point
At present, there is no evidence that any of the filters used in sunscreens have harmful effects in children. However, the use of organic filters such as oxybenzone and octocrylene, as well as the nanoparticles found in inorganic filters, is controversial, especially in young children and in patients with damage to the stratum corneum.
Vitamin D is a hormone that is fundamental to the adequate development and maintenance of the musculoskeletal system. It also plays an important role in other functions of the organism, for example in the immune and cardiovascular systems.52 Epidemiologic studies carried out in adults in places such as Australia, Bangladesh, and Hong Kong suggest that the effect of skin cancer prevention practices on vitamin D serum levels might depend on the sun protection measures used, place of residence, skin phototype, age, and the quantity of vitamin D ingested orally. Despite the high year-round levels of solar irradiance in the aforementioned locations, the studies found suboptimal mean vitamin D serum levels in adults.54
The currently available evidence has not shown that sunscreen use affects vitamin D levels in the general population.55 Regular failure to implement the theoretical requirements for adequate sunscreen application can, in fact, favor the production of vitamin D in many individuals.56 It has been shown that vitamin D production increases exponentially in response to UV-B exposure when the layer of sunscreen applied is thinner than recommended (<2mg cm-2).57 Unfortunately, no specific studies have been carried out in children.
Although vitamin D is undoubtedly important to maintaining good health, it would be inappropriate to recommend that it be obtained by means of intentional sun exposure because UV radiation is known to be carcinogenic to humans.58 It has been established that the effective UV radiation dose to produce 1000 IU of vitamin D—the dose that guarantees sufficiently high serum levels for the vitamin to perform its functions—is 25% of the minimal erythema dose of incident UV radiation on 25% of the body surface area (hands, arms, and face). In addition, solar radiation must be weighted according to the moment at which it is measured.53 Sun exposure outside of peak sun times (10:00 am to 3:00 pm in the spring, summer, and fall) has a limited impact on the cutaneous synthesis of vitamin D.59 Moreover, during the winter months, very few UV-B photons are able to reach the surface of the earth at latitudes higher than 35°. No studies in children have identified a level of sun exposure at which dietary vitamin D intake might no longer be necessary. Both the American Academy of Pediatrics and the nutrition committee of the Spanish Association of Pediatrics (AAP) recommend the use of dietary vitamin D3 supplements at a daily dose of 400 IU from birth until the age of 1 year or the use of sufficiently vitamin D–enriched infant formulas.60 Children over the age of 1 year and adolescents whose daily dietary intake of vitamin D does not reach 400 IU are also advised to take supplements at this dose.
Do Current Sun Protection Strategies Meet Children's Sun Protection Needs?Sun Protection CampaignsAs in any aspect of health, the most appropriate long-term strategy for achieving adequate, responsible sun exposure is to raise awareness and encourage the population to adopt healthy habits. Because it is preferable that these habits be adopted during childhood, these messages should be transmitted through health-education campaigns that show children how to make these habits part of their routine. These campaigns must be coordinated with other efforts to promote healthy behaviors, such as campaigns to prevent obesity and metabolic syndrome. It has been shown that the promotion of sun protection habits does not lead to an increase in sedentary habits or body mass in children. Ideally, these campaigns should be orchestrated in a coordinated manner and public health organizations should dedicate sufficient resources to them. Perhaps counterintuitively, the advice given by pediatricians or dermatologists, albeit useful, does little to modify the habits of the population.61 Large-scale campaigns such as SunSmart in Australia,62 SunSafe and SunWise in the United States,63–65 and, on a smaller scale, SolSano in Spain66 aim to teach children and their parents how to avoid the harmful effects of sun exposure while engaging in outdoor activities.
General Sun Protection MeasuresSun protection measures that are advisable for all children, regardless of skin phototype, include wearing clothing and hats, limiting sun exposure during midday hours, and, for children over the age of 6 months, regularly using sunscreens with an SPF of at least 15.67
Sunscreen UseAs explained above, because of the immaturity of the epidermal barrier and the prevalence of atopic dermatitis, especially in very young children, it is especially important that children's sunscreens be minimally irritating, with little or no potential for sensitization, and that they not be absorbed by the skin. Because children tend to rub their faces, it is also important that children's sunscreens not be irritating to the eyes. In addition, it is fundamental that children use sunscreens that offer broad-spectrum protection. To meet the regular conditions of use in children, they should also be long-lasting, water-resistant, and able to withstand physical activity. The question is whether sunscreens designed for children meet these requirements.
It may come as a surprise that the European Commission Recommendation of 22 September 2006 does not establish any obligatory requirements for children's sunscreens. In general, sunscreens for children contain the same types of filters as those designed for adults. Some children's sunscreens have the same characteristics as sunscreens for sensitive skin and most are resistant or very resistant to water (able to withstand 2-4 immersions of 20minutes, as required by law).
Oil-based emulsions containing inorganic filters appear to be the most appropriate sunscreens for preventing sensitization, irritation, and photoallergy, but these products have not been tested in children.4 It has been demonstrated, however, that only children's sunscreens containing both organic and inorganic filters can provide an SPF of 50; sunscreens containing only inorganic filters cannot offer this level of protection.68
Application of a sufficient amount of sunscreen is an important factor in the effectiveness of these products. Adults tend to apply between one-quarter and one-half of the recommended amount (2mg/cm2), and the SPF decreases in proportion to the amount applied. A recent study showed that the same effect occurs when children apply sunscreen on their own.69 The average amount of sunscreen applied by children between the ages of 5 and 12 years was 0.48mg/cm2. The amount applied was greater if the container was a pump dispenser than if it was a squeeze bottle or a roll-on container. Children aged 5 to 7 years tended to apply larger amounts of sunscreen (0.62-0.65mg/cm2) than those aged 10 to 12 years (0.28-0.37mg/cm2).
Recommendations for Adequate Sun Protection in ChildrenGiven the considerations set out above, in accordance with the recommendations of the American Academy of Pediatrics38 and of the American Academy of Dermatology in 2012, we recommend that children and adolescents follow the sun protection advice presented in Table 2.
Sun Protection Measures for Children and Adolescents.
| • Avoidance of midday sun and wearing clothing, hats, and sunglasses. |
| • Use of sunscreens with the following characteristics: |
| SPF ≥ 30 (reapply every 2h). |
| Broad protection spectrum (label should show the word “UVA” with a circle around it). |
| Water resistance. |
| Oxybenzone-free (and octocrylene-free, if possible, especially if used in atopic children). |
| • Special caution in children with nevi, freckles, or a family history of melanoma. |
| • Emphasis on sun protection measures in children older than 10 years, the age at which the use of these measures decreases sharply as interest in tanning increases. |
| • Avoidance of UV-A radiation during adolescence. |
| • Although sun exposure is a source of vitamin D, administration of 400 IU of vitamin D3 to infants under the age of 1 year and older children and adolescents whose daily dietary intake of vitamin D is lower than 400 IU. |
| • Sun protection recommendations for children under the age of 2 years: |
| Avoidance of sun exposure. |
| Use of light, tightly woven clothing. |
| Application of sunscreen, when necessary, only to areas not covered by clothing or a hat. |
Given the unique anatomical, physiological, and functional characteristics of children's skin, the available evidence reviewed in this article supports the promotion of specific sun protection strategies for children. These strategies are also advisable because of the carcinogenic effects that exposure to UV radiation during childhood presumably have later in life. Nevertheless, much more evidence is needed. Ideally, we need to know the requirements and needs for each age range because it is not very realistic to consider all of childhood as a single age range. Additionally, sun protection strategies should be personalized according to skin phototype, latitude, and exposure habits.
Sunscreens are the most commonly used sun protection measure. Sunscreens marketed for children generally only highlight some of the characteristics also found in adult sunscreens: high protection level, resistance to water, ability to withstand physical activity, and predominately inorganic filters.
There is a need for child-specific sun protection strategies. However, our technical capacity to address this need is limited by our incomplete understanding of the specific nature of actinic damage during childhood. As a result, current regulations only establish generic limits. The existence of specific children's sunscreens is justified, not only to highlight the special importance of sun protection in children but also to offer products with differentiated, age-appropriate characteristics.
Until more is known about the spectrum of actinic damage in children from both an epidemiologic and a pathophysiological point of view, the most appropriate strategies—and also the most difficult to implement—are likely to involve prevention campaigns and efforts to encourage the general population, and children in particular, to adopt healthy sun protection habits.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentialityThe authors declare that they have followed the protocols of their hospitals concerning the publication of patient data and that all patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gilaberte Y, Carrascosa J.M. Realidades y retos de la fotoprotección en la infancia. Actas Dermosifiliogr. 2014;105:253–262.